Abstract
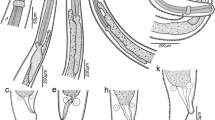
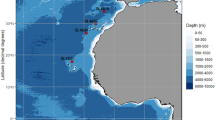
The morphological and molecular identification of Hysterothylacium larval morphotypes in the Black Sea remains unresolved and incomplete. The aim of current study was to provide a detailed morphological identification with rDNA whole ITS (ITS‒1, 5.8S subunit, ITS‒2) and mtDNA cox2 sequences data of Hysterothylacium larval morphotypes infecting four commonly edible marine fish species, including European anchovy, Engraulis encrasicolus (L.), horse mackerel, Trachurus trachurus (L.), whiting, Merlangius merlangus (L.), and red mullet, Mullus barbatus ponticus (E.) in the Black Sea (FAO fishing area 37.4.2). Hysterothylacium larval morphotypes were morphologically classified, followed by whole ITS and cox2 sequencing. Four Hysterothylacium larval morphotypes, III, IV, VIII, and IX, are described based on morphological and molecular data. The present study provides the first study reporting whole ITS and cox2 sequences for Hysterothylacium larval morphotypes III, IV, VIII and III, IV, VIII, IX, respectively, in the Black Sea. Here, we offer a foundation for future research on the distribution, morphologic and molecular identification of Hysterothylacium larval morphotypes infecting edible some marine fish in the Black Sea.






Similar content being viewed by others
Data availability
Data supporting the conclusions of this article are included within the article. Nucleotide sequences generated in this study were deposited in the GenBank database under the accession numbers MZ313180‒MZ313183 and MZ313477‒ MZ313488.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Cannon LRG (1977) Some larval ascaridoids from south-eastern Queensland marine fishes. Int J Food Microbiol 7:233–243
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552
Costa A, Graci S, Cammilleri G, Buscemi MD, Collura R, Vella A, Ferrantelli V (2018) Molecular identification of Hysterothylacium spp. in fishes from the Southern Mediterranean Sea (Southern Italy). J Parasitol 104:398–406
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Ghadam M, Banaii M, Mohammed ET, Suthar J, Shamsi S (2018) Morphological and molecular characterization of selected species of Hysterothylacium (Nematoda: Raphidascarididae) from marine fish in Iraqi waters. J Helminthol 92:116–124
González-Amores Y, Clavijo-Frutos E, Salas-Casanova C, Alcain-Martínez G (2015) Direct parasitologial diagnosis of infection with Hysterothylacium aduncum in a patient with epigastralgia. Rev Esp Enferm Dig 107:699–700
Guo YN, Xu Z, Zhang LP, Hu YH, Li L (2014) Occurrence of Hysterothylacium and Anisakis nematodes (Ascaridida: Ascaridoidea) in the tanaka’s snailfish Liparis tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae). Parasitol Res 113:1289–1300
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192
Hossen MS, Shamsi S (2019) Zoonotic nematode parasites infecting selected edible fish in New South Wales, Australia. Int J Food Microbiol 308:108306
Hossen MS, Wassens S, Shamsi S (2021) Integrative species delimitation and community structure of nematodes in three species of Australian flathead fishes (Scorpaeniformes: Platycephalidae). Parasitol Res 120:461–480
Hossen MS, Suthar J, Wassens S, Shamsi S (2023) Occurrence and molecular identification of nematodes from blue mackerel Scomber australasicus Cuvier in Australian waters. Parasitol Int 92:102664
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Khammassi M, Bahri S, Pekmezci GZ (2020) Morphological and molecular identification of Hysterothylacium larvae (Nematoda: Raphidascarididae) in marine fish from Tunisian Mediterranean coasts. Parasitol Res 119: 3285–3296
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Nadler SA, Hudspeth DS (2000) Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: hypotheses of structural and sequence evolution. J Parasitol 86:380–393
Ozer A, Kornyychuk YM, Yurakhno V, Ozturk T (2016) Seasonality and host-parasite interrelationship of Hysterothylacium aduncum (Nematoda) in whiting Merlangius merlangus off the southern and northern coasts of the Black Sea. Helminthologia 53(3):248–256
Ozturk T, Yesil A (2018) Metazoan parasite fauna of the red mullet, Mullus barbatus ponticus Essipov, 1927 in the Sinop coasts of the Black Sea. Turk J Fish Aquat Sci 18(1):153–160
Pantoja CS, Pereira FB, Santos CP, Luque JL (2016) Morphology and molecular characterization hold hands: clarifying the taxonomy of Hysterothylacium (Nematoda: Anisakidae) larval forms. Parasitol Res 115:4353–4364
Pekmezci GZ, Bolukbas CS, Gurler AT, Onuk EE (2013) Occurrence and molecular characterization of Hysterothylacium aduncum (Nematoda: Anisakidae) from Merlangius merlangus euxinus and Trachurus trachurus off the Turkish coast of Black Sea. Parasitol Res 112:1031–1037
Pekmezci GZ, Yardimci B, Onuk EE, Umur S (2014) Molecular characterization of Hysterothylacium fabri (Nematoda: Anisakidae) from Zeus faber (Pisces: Zeidae) caught off the Mediterranean coasts of Turkey based on nuclear ribosomal and mitochondrial DNA sequences. Parasitol Int 63:127–131
Petter AJ, Maillard C (1987) Ascarides de Poissons de Méditerranée occidentale. Bull Mus Natl Hist Nat 9:773–798
Reiczigel J, Marozzi M, Fábián I, Rózsa L (2019) Biostatistics for parasitologists – a primer to quantitative parasitology. Trends Parasitol 35:277–281
Roca-Geronès X, Montoliu I, Godínez-González C, Fisa R, Shamsi S (2018) Morphological and genetic characterization of Hysterothylacium Ward & Magath, 1917 (Nematoda: Raphidascarididae) larvae in horse mackerel, blue whiting and anchovy from Spanish Atlantic and Mediterranean waters. J Fish Dis 41:1463–1475
Roca-Geronès X, Segovia M, Godínez-González C, Fisa R, Montoliu I (2020) Anisakis and Hysterothylacium species in Mediterranean and North-East Atlantic fishes commonly consumed in Spain: Epidemiological, molecular and morphometric discriminant analysis. Int J Food Microbiol 325:108642
Rózsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86:228–232
Shamsi S, Eisenbarth A, Saptarshi S, Beveridge I, Gasser RB, Lopata AL (2011) Occurrence and abundance of anisakid nematode larvae in five species of fish from southern Australian waters. Parasitol Res 108(4):927–934
Shamsi S (2017) Morphometric and molecular descriptions of three new species of Hysterothylacium (Nematoda: Raphidascarididae) from Australian marine fish. J Helminthol 91:613–624
Shamsi S, Suthar J (2016) A revised method of examining fish for infection with zoonotic nematode larvae. Int J Food Microbiol 227:13–16
Shamsi S, Gasser R, Beveridge I (2013) Description and genetic characterisation of Hysterothylacium (Nematoda: Raphidascarididae) larvae parasitic in Australian marine fishes. Parasitol Int 62:320–328
Shamsi S, Poupa A, Justine JL (2015) Characterisation of Ascaridoid larvae from marine fish off New Caledonia, with description of new Hysterothylacium larval types XIII and XIV. Parasitol Int 64:397–404
Shamsi S, Ghadam M, Suthar J, Ebrahimzadeh Mousavi H, Soltani M, Mirzargar S (2016) Occurrence of ascaridoid nematodes in selected edible fish from the Persian Gulf and description of Hysterothylacium larval type XV and Hysterothylacium persicum n. sp. (Nematoda: Raphidascarididae). Int J Food Microbiol 236:65–73
Shamsi S, Steller E, Chen Y (2018) New and known zoonotic nematode larvae within selected fish species from Queensland waters in Australia. Int J Food Microbiol 272:73–82
Suthar J, Shamsi S (2021) The occurrence and abundance of infective stages of zoonotic nematodes in selected edible fish sold in Australian fish markets. Microbial Pathogenesis 154:104833
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Yagi K, Nagasawa K, Ishikura H, Nakagawa A, Sato N, Kikuchi K, Ishikura H (1996) Female worm Hysterothylacium aduncum excreted from human: a case report. Jpn J Parasitol 45:12–23
Zhu X, Gasser RB, Podolska M, Chilton NB (1998) Characterization of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. Int J Parasitol 28:1911–1921
Funding
This study was supported by the Scientific Research Council of Ondokuz Mayis University, Samsun, Türkiye [grant number PYO.VET.1904.20.022].
Author information
Authors and Affiliations
Contributions
G.Z.P. supervised and coordinated the present study. G.Z.P. came up with the study idea and drafted the initial proposal. G.Z.P. and M.Y.G. carried out the laboratory analyses. G.Z.P. and M.Y.G. have also read and approved the final manuscript. This work is also summarized from the master thesis of M.Y.G., Ondokuz Mayis University, Samsun, Türkiye, in 2021.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict/competing interests.
Additional information
Section Editor: Guillermo Salgado-Maldonado
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gelen, M.Y., Pekmezci, G.Z. Morphological and molecular characterization of Hysterothylacium larval morphotypes (Nematoda: Raphidascarididae) infecting edible marine fish in the Black Sea. Parasitol Res 122, 1863–1872 (2023). https://doi.org/10.1007/s00436-023-07887-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07887-3