Abstract
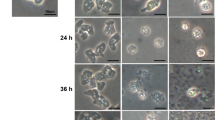
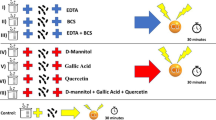
Free-living amoebae belonging to the genus Acanthamoeba are the causative agents of infections in humans and animals. Many studies are being conducted to find effective compounds against amoebae, but their sublethal concentration effects on surviving amoebae seem to have been overlooked. Chlorine is a common disinfection agent commonly added to public water facilities and supplies. In this study, the cytopathic and phagocytic properties of Acanthamoeba castellanii trophozoites following exposure to sublethal concentrations of chlorine were examined. Two hours of exposure to 5 ppm hypochlorite calcium was considered the sublethal concentration for A. castellanii trophozoites. To compare the pathogenic potential of treated and untreated Acanthamoeba trophozoites, cytotoxicity, adhesion assays in RAW 264.7 macrophages, osmo, and thermotolerance tests were carried out. Bacterial uptake was assessed in treated cells to evaluate their phagocytic characteristics. Oxidative stress biomarkers and antioxidant activities were compared in treated and untreated trophozoites. Finally, the mRNA expression of the mannose-binding protein (MBP), cysteine protease 3 (CP3), and serine endopeptidase (SEP) genes was determined in cells. In all the experiments, untreated trophozoites were considered the control. In comparison to untreated trophozoites, in chlorine-treated trophozoites, cytopathic effects were more extensive and resulted in the detachment of macrophage monolayers. Treated trophozoites could not grow at high temperatures (43 °C). Besides, they showed osmotolerance to 0.5 M D-mannitol but not to 1 M. Results demonstrated a higher bacterial uptake rate by chlorine-treated trophozoites than untreated cells. The treated and untreated cells had significantly different glutathione and glutathione/glutathione disulfide ratios. Antioxidant enzyme activities, total antioxidant capacity, and malondialdehyde levels were increased significantly in chlorine-treated cells. Quantifying mRNA expression in chlorine-treated trophozoites revealed that virulence genes were upregulated. Chlorine can form resistance and virulent amoebae if it is not used at a proper concentration and exposure time. Identification of stress responses, their mechanisms in Acanthamoeba, and their relation to amoeba virulence would give us a better perception of their pathophysiology.



Similar content being viewed by others
Availability of data and materials
The data presented in this study are available within the article.
References
Alsam S, Sissons J, Dudley R, Khan NA (2005) Mechanisms associated with Acanthamoeba castellanii (T4) phagocytosis. Parasitol Res 96:402–409
Anwar A, Rajendran K, Siddiqui R, Raza Shah M, Khan NA (2018) Clinically approved drugs against CNS diseases as potential therapeutic agents to target brain-eating amoebae. ACS Chem Neurosci 10:658–666
Asadi Z, Bahrami S, Zarei M, Hamidinejat H (2022) Effect of chlorine on survival and ultrastructural changes of Acanthamoeba castellanii. J Appl Biol (accepted and in press)
Bahrami S, Zarei M, Liljebjelke K (2022) Chlorine-stressed Salmonella cells are significantly more engulfed by Acanthamoeba trophozoites and have a longer intracystic survival than the non-stressed cells. Food Microbiol 102:103927
Baldevraj RM, Jagadish RS (2011) Incorporation of chemical antimicrobial agents into polymeric films for food packaging. In: Multifunctional and Nanoreinforced Polymers for Food Packaging. Woodhead Publishing, Sawston, UK, pp 368–420
Benzie IF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Brown SD, Gauthier TW, Brown LA (2009) Impaired terminal differentiation of pulmonary macrophages in a guinea pig model of chronic ethanol ingestion. Alcohol Clin Exp Res 33:1782–1793
Bowers B, Olszewski TE (1983) Acanthamoeba discriminates internally between digestible and indigestible particles. J Cell Biol 97:317–322
Chaúque BJ, Dos Santos DL, Anvari D, Rott MB (2022) Prevalence of free-living amoebae in swimming pools and recreational waters, a systematic review and meta-analysis. Parasitol Res 121(11):3033–3050
Chaves RD, Aspridou Z, Sant’Ana AS, Koutsoumanis KP (2019) Effect of chlorine stress on the subsequent growth behavior of individual Salmonella cells. Food Res Int 123:311–316
Corsaro D, Köhsler M, Montalbano Di Filippo M, Venditti D, Monno R, Di Cave D, Berrilli F, Walochnik J (2017) Update on Acanthamoeba jacobsi genotype T15, including full-length 18S rDNA molecular phylogeny. Parasitol Res 116:1273–1284
Dhakal J, Sharma CS, Nannapaneni R, Mc Daniel CD, Kim T, Kiess A (2019) Effect of chlorine-induced sublethal oxidative stress on the biofilm-forming ability of Salmonella at different temperatures, nutrient conditions, and substrates. J Food Protect 82(1):78–92
Dudley R, Alsam S, Ahmed Khan N (2008) The role of proteases in the differentiation of Acanthamoeba castellanii. FEMS Microbiology Lett 286:9–15
Finnegan M, Linley E, Denyer SP, McDonnell G, Simons C, Maillard JY (2010) Mode of action of hydrogen peroxide and other oxidizing agents: differences between liquid and gas forms. J Antimicrob Chemother 65:2108–2115
Galal-Gorchev H (1996) Chlorine in water disinfection. Pure Appl Chem 68(9):1731–1735
German N, Doyscher D, Rensing C (2013) Bacterial killing in macrophages and amoeba: do they all use a brass dagger? Future Microbiol 8:1257–1264
Gomes IB, Simões M, Simões LC (2016) The effects of sodium hypochlorite against selected drinking water-isolated bacteria in planktonic and sessile states. Sci Total Environ 565:40–48
González-Robles A, Salazar-Villatoro L, Omaña-Molina M, Reyes-Batlle M, Martín-Navarro CM, Lorenzo-Morales J (2014) Morphological features and in vitro cytopathic effect of Acanthamoeba griffini trophozoites isolated from a clinical case. J Parasitol Res 1:2014
Jeong HJ, Jang ES, Han BI, Lee KH (2007) Acanthamoeba: could it be an environmental host of Shigella? Exp Parasitol 115:181–186
Jimenez V (2014) Dealing with environmental challenges: mechanisms of adaptation in Trypanosoma cruzi. Res Microbiol 165:155–165
Jones DP, Go YM (2010) Redox compartmentalization and cellular stress. Diabetes Obes Metab 12:116–125
Khan NA (2001) Pathogenicity, morphology, and differentiation of Acanthamoeba. Curr Microbiol 43(6):391–395
Korolyuk MA, Ivanova LI, Maiorova IG, Tokarev VE (1988) A method for measuring catalase activity. Lab Delo 1:16–19
Krishnan K, Askew DS (2014) Endoplasmic reticulum stress and fungal pathogenesis. Fungal. Biol Rev 28(2-3):29–35
Landell MF, Salton J, Caumo K, Broetto L, Rott MB (2013) Isolation and genotyping of free-living environmental isolates of Acanthamoeba spp. from bromeliads in Southern Brazil. Exp Parasitol 134(3):290–294
Liu X, Liu F, Ding S, Shen J, Zhu K (2020) Sublethal levels of antibiotics promote bacterial persistence in epithelial cells. Adv Sci 7(18):1900840
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16:273–307
Martín-Navarro CM, Lorenzo-Morales J, Machín RP, López-Arencibia A, Valladares B, Piñero JE (2010) Acanthamoeba spp.: in vitro effects of clinical isolates on murine macrophages, osteosarcoma and HeLa cells. Exp parasitol 126:85–88
Motavallihaghi S, Khodadadi I, Goudarzi F, Afshar S, Shahbazi AE, Maghsood AH (2022) The role of Acanthamoeba castellanii (T4 genotype) antioxidant enzymes in parasite survival under H2O2-induced oxidative stress. Parasitol Int 87:102523
Montalbano Di Filippo M, Santoro M, Lovreglio P, Monno R, Capolongo C, Calia C, Fumarola L, D’Alfonso R, Berrilli F, Di Cave D (2015) Isolation and molecular characterization of free-living amoebae from different water sources in Italy. Int J Environ Res. Public Health 12:3417–3427
Moon EK, Chung DI, Hong YC, Kong HH (2008) Characterization of a serine proteinase mediating encystation of Acanthamoeba. Eukaryot Cell 7:1513–1517
Mungroo MR, Siddiqui R, Khan NA (2021) War of the microbial world: Acanthamoeba spp. interactions with microorganisms. Folia Microbiol 66:689–699
Neelam S, Niederkorn JY (2017) Pathobiology and immunobiology of Acanthamoeba keratitis: insights from animal models. Yale J Biol Med 90:261–268
Ng SL, Nordin A, Abd Ghafar N, Suboh Y, Ab Rahim N, Chua KH (2017) Acanthamoeba-mediated cytopathic effect correlates with MBP and AhLBP mRNA expression. Parasit Vectors 10:1–4
Placer ZA, Crushman LL, Johnson BC (1966) Estimation of products of lipid peroxidation (MDA) in biochemical systems. Anal Chem 16:359–364
Roberts TA, Cordier JL, Gram L, Tompkin RB, Pitt JI, Gorris LGM, Swanson KMJ (eds) (2005) Microorganisms in foods 6: microbial ecology of food commodities. Springer, US, London, UK
Scheid P (2018) Free-living amoebae as human parasites and hosts for pathogenic microorganisms. Proceedings 2:692
Sissons J, Alsam S, Jayasekera S, Kim KS, Stins M, Khan NA (2004) Acanthamoeba induces cell-cycle arrest in host cells. J Med Microbiol 53:711–717
Suresh D, Annam V, Pratibha K, Maruti Prasad BV (2009) Total antioxidant capacity – a novel early bio-chemical marker of oxidative stress in HIV infected individuals. J Biomed Sci 16:61
Torno MS, Babapour R, Gurevitch A, Witt MD (2000) Cutaneous acanthamoebiasis in AIDS. J Am Acad Dermatol 42:351–354
Wang S, Phillippy AM, Deng K, Rui X, Li Z, Tortorello ML, Zhang W (2010) Transcriptomic responses of Salmonella enterica Serovars Enteritidis and Typhimurium to chlorine-based oxidative stress. Appl Environ Microbiol 76:5013–5024
Wang Z, Wu D, Tachibana H, Feng M, Cheng XJ (2020) Identification and biochemical characterisation of Acanthamoeba castellanii cysteine protease 3. Parasites & Vectors 13:1–11
Yamakura F, Kawasaki H (2010) Post-translational modifications of superoxide dismutase. Biochim Biophys Acta 1804:318–325
Zarei M, Eskandari MH, Keshtkaran S (2014) Survival of normal and chlorine-stressed pathogenic and non-pathogenic Vibrio parahaemolyticus under adverse conditions. Jundishapur J Microbiol 7(3):e9313
Zarei M, Ghahfarokhi ME, Fazlara A, Bahrami S (2019) Effect of the bacterial growth phase and co-culture conditions on the interaction of Acanthamoeba castellanii with Shigella dysenteriae, Shigella flexneri, and Shigella sonnei. J Basic Microbiol 59:735–743
Acknowledgement
This study was supported by the research grant provided by the Shahid Chamran University of Ahvaz.
Author information
Authors and Affiliations
Contributions
S.B., M.Z., and F.L.H. designed the research; S.B., M.Z., and Z.A. performed the experiments. Z.A., S.B., and H.H. performed data analysis and prepared the figures; S.B. wrote the manuscript; F.L.H. revised the manuscript; S.B., Z.A., M.Z., H.H., and F.L.H. approved the version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Sutherland Maciver
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bahrami, S., Asadi, Z., Zarei, M. et al. Exposure to sublethal concentrations of chlorine enhances the cytotoxicity of Acanthamoeba castellanii. Parasitol Res 122, 1371–1380 (2023). https://doi.org/10.1007/s00436-023-07837-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07837-z