Abstract
The protozoan parasite Neospora caninum infects carnivores as definitive and a wide range of mammals as intermediate hosts. This parasite is regarded as an important cause of abortion in cattle worldwide, causing significant economic losses. Although there is serological evidence of infection in Old World camelids, the significance of N. caninum in these animal species is still poorly understood. The aim of this study was to use molecular and histological methods to detect N. caninum in the blood and tissues of 100 slaughtered one-humped camels (Camelus dromedarius) in Iran. For this, genomic DNA was extracted from blood, brain, portal lymph node and liver of the camels, and nested-PCR assay followed by sequencing were performed. Besides, paraffin-embedded tissue sections were stained with hematoxylin and eosin (H&E) and studied microscopically. In addition, immunohistochemical staining for N. caninum was attempted on brain samples with positive PCR results. All animals were tested for antibodies against N. caninum and Toxoplasma gondii by whole tachyzoite-agglutination tests. N. caninum DNA was detected in blood, brain, and portal lymph node, but not in the liver of two (2%) camels. Histopathological examination revealed cysts resembling N. caninum in brain samples of one of these camels; however, immunohistochemical staining for N. caninum and T. gondii did not allow a morphological identification. IgG antibodies to N. caninum and T. gondii were detected in 36% and 35% of the camels, respectively. This study provides the first insight into direct detection of N. caninum in C. dromedarius in Iran. Further molecular studies on aborted fetuses, stillborn animals and cases of perinatal mortality are needed to understand the possible involvement of N. caninum in cases of reproductive failure. As the definitive hosts of N. caninum are domestic and wild canids, producers should be advised to monitor and limit exposure of their camelids to these species and their feces.
Highlights
-
First direct detection of N. caninum DNA in blood and tissues of one-humped camels (Camelus dromedarius) in Iran.
-
N. caninum DNA was detected in blood, brain, portal lymph node, but not liver tissue.
-
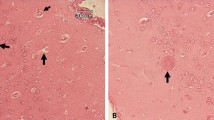
Tissue cysts were observed in histological sections of the brain in one camel.

Similar content being viewed by others
Data availability
Data supporting the conclusions of this article are included within the article and its supplementary tables.
References
Agriculture-Jahad Ministry of Iran (2020) Annual production report [in Persian]. Tehran, Iran
Al-Anazi AD (2011) Prevalence of Neospora caninum and Toxoplasma gondii antibodies in sera from camels (Camelus dromedarius) in Riyadh Province, Saudi Arabia. J Egypt Soc Parasitol 41:245–250
Amdouni Y, Abedennebi I, Amairia S et al (2022) First molecular detection of Neospora caninum from naturally infected slaughtered camels in Tunisia. Vet Med Sci 8(5):2241–2247. https://doi.org/10.1002/vms3.901
Bártová E, Kobédová K, Lamka J et al (2017) Seroprevalence of Neospora caninum and Toxoplasma gondii in exotic ruminants and camelids in the Czech Republic. Parasitol Res 116:1925–1929
Basso W, Sollberger E, Schares G et al (2020) Toxoplasma gondii and Neospora caninum infections in South American camelids in Switzerland and assessment of serological tests for diagnosis. Parasit Vectors 13:256
Bień J, Moskwa B, Cabaj W (2010) In vitro isolation and identification of the first Neospora caninum isolate from European bison (Bison bonasus bonasus L.). Vet Parasitol 173:200–205
Buxton D, Maley S, Wright S et al (1998) The pathogenesis of experimental neosporosis in pregnant sheep. J Comp Pathol 118:267–279
Calero-Bernal R, Horcajo P, Hernández M et al (2019) Absence of Neospora caninum DNA in human clinical samples, Spain. Emerg Infect Dis 25:1226–1227
Diall O, Desquesnes M, Faye B et al (2022) Development of a control strategy towards elimination of Trypanosoma evansi infection (surra) in camels in Africa. Acta Trop 234:106583. https://doi.org/10.1016/j.actatropica.2022.106583
Donahoe SL, Lindsay SA, Krockenberger M et al (2015) A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int J Parasitol Parasites Wildl 4:216–238
Dubey JP, Desmonts G (1987) Serological responses of equids fed Toxoplasma gondii oocysts. Equine Vet J 19:337–339
Dubey JP, Hemphill A, Calero-Bernal R, Schares G (2017) Neosporosis in animals. CRC Press, Boca Raton, FL, USA
Dubey JP, Hemphill A, Calero-Bernal R, Schares G (2017) Neosporosis in camels and south American camelids. In: Dubey JP, Hemphill A, Calero-Bernal R, Schares G (eds) Neosporosis in Animals. CRC Press, Boca Raton, USA, pp 347–350
Dubey JP, Jenkins M, Rajendran C et al (2011) Gray wolf (Canis lupus) is a natural definitive host for Neospora caninum. Vet Parasitol 181:382–387
Dubey JP, Liddell S, Mattson D et al (2001) Characterization of the Oregon isolate of Neospora hughesi from a horse. J Parasitol 87:345–353
Dubey JP, Sreekumar C, Knickman E et al (2004) Biologic, morphologic, and molecular characterisation of Neospora caninum isolates from littermate dogs. Int J Parasitol 34:1157–1167
FAOSTAT (2022) Statistics division of food and agriculture organization of the united nations. https://www.fao.org/faostat/en/#data. Accessed 17 Jan 2023
Ferre I, Aduriz G, del-Pozo I, et al (2005) Detection of Neospora caninum in the semen and blood of naturally infected bulls. Theriogenology 63:1504–1518
Gharekhani J, Yakhchali M, Berahmat R (2020) Neospora caninum infection in Iran (2004–2020): a review. J Parasit Dis 44(4):671–686. https://doi.org/10.1007/s12639-020-01266-w
Gondim LF, Laski P, Gao L, McAllister MM (2004) Variation of the internal transcribed spacer 1 sequence within individual strains and among different strains of Neospora caninum. J Parasitol 90:119–122
Goodswen SJ, Kennedy PJ, Ellis JT (2013) A review of the infection, genetics, and evolution of Neospora caninum: from the past to the present. Infect Genet Evol 13:133–150
Hamidinejat H, Ghorbanpour M, Rasooli A et al (2013) Occurrence of anti-Toxoplasma gondii and Neospora caninum antibodies in camels (Camelus dromedarius) in the center of Iran. Turk J Vet Anim Sci 37:277–281. https://doi.org/10.3906/vet-1110-21
Hilali M, Romand S, Thulliez P et al (1998) Prevalence of Neospora caninum and Toxoplasma gondii antibodies in sera from camels from Egypt. Vet Parasitol 75:269–271
Ho M, Barr BC, Marsh AE et al (1996) Identification of bovine Neospora parasites by PCR amplification and specific small-subunit rRNA sequence probe hybridization. J Clin Microbiol 34:1203–1208
Hosseininejad M, Pirali Kheirabadi K, Hosseini F (2009) Seroprevalence of Neospora caninum infection in camels (Camelus dromedarius) in Isfahan Province, center of Iran. Iran J Parasitol 4:61–64
Lindsay DS, Dubey J (2020) Neosporosis, toxoplasmosis, and sarcocystosis in ruminants: an update. Vet Clin Food Anim Pract 36:205–222
McInnes LM, Ryan UM, O’Handley R et al (2006) Diagnostic significance of Neospora caninum DNA detected by PCR in cattle serum. Vet Parasitol 142:207–213. https://doi.org/10.1016/j.vetpar.2006.07.013
Nakagaki KY, Abreu CC, Costa RC et al (2016) Lesions and distribution of Neospora caninum in tissues of naturally infected female goats. Small Rumin Res 140:57–62
Nazir MM, Oneeb M, Ayaz MM et al (2017) Prevalence of antibodies to Neospora caninum in the serum of camels (Camelus dromedarius) from central Punjab, Pakistan. Trop Anim Health Prod 49:1081–1084
Okeoma C, Williamson N, Pomroy W et al (2004) The use of PCR to detect Neospora caninum DNA in the blood of naturally infected cows. Vet Parasitol 122:307–315
Packham AE, Sverlow KW, Conrad PA et al (1998) A modified agglutination test for Neospora caninum: development, optimization, and comparison to the indirect fluorescent-antibody test and enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 5:467–473
Pearson LK, Rodriguez JS, Tibary A (2014) Disorders and diseases of pregnancy. In: Cebra C, Anderson DE, Tibary A, et al. (eds) Llama and Alpaca Care. Elsevier, St. Louis, USA, 256–273
Piergili Fioretti D, Pasquali P, Diaferia M et al (2003) Neospora caninum infection and congenital transmission: serological and parasitological study of cows up to the fourth gestation. J Vet Med Ser B 50:399–404. https://doi.org/10.1046/j.1439-0450.2003.00686.x
Sadrebazzaz A, Haddadzadeh H, Shayan P (2006) Seroprevalence of Neospora caninum and Toxoplasma gondii in camels (Camelus dromedarius) in Mashhad. Iran Parasitol Res 98:600–601. https://doi.org/10.1007/s00436-005-0118-3
Sazmand A, Joachim A (2017) Parasitic diseases of camels in Iran (1931–2017)- a literature review. Parasite 24:21. https://doi.org/10.1051/parasite/2017024
Serrano-Martínez E, Collantes-Fernández E, Chávez-Velásquez A et al (2007) Evaluation of Neospora caninum and Toxoplasma gondii infections in alpaca (Vicugna pacos) and llama (Lama glama) aborted foetuses from Peru. Vet Parasitol 150:39–45. https://doi.org/10.1016/j.vetpar.2007.08.048
Serrano-Martínez E, Collantes-Fernández E, Rodríguez-Bertos A et al (2004) Neospora species-associated abortion in alpacas (Vicugna pacos) and llamas (Llama glama). Vet Rec 155:748–749
Winzer P, Anghel N, Imhof D et al (2020) Neospora caninum: structure and fate of multinucleated complexes induced by the bumped kinase inhibitor BKI-1294. Pathogens 9:382
Funding
This study was funded by Bu-Ali Sina University, Hamedan, Iran, under the framework of Mr. Saeid Karimi M.Sc. thesis.
Author information
Authors and Affiliations
Contributions
Saeid Karimi: investigation, methodology. Aliasghar Bahari: conceptualization, investigation, methodology, resources, data curation, validation, supervision, project administration, funding acquisition. Alireza Nourian: investigation, methodology, writing—original draft. Sakineh Azami: investigation, methodology, writing—original draft. Mehdi Namavari: investigation, methodology, writing—original draft. Alireza Sazmand: investigation, data curation, validation, writing—original draft, writing—review and editing. Andrew Hemphill, Walter Basso: investigation, writing—original draft, writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Sampling was approved by the Research Council of Faculty of Veterinary Science, Bu‐Ali Sina University, Iran.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Section Editor: Sutherland Maciver
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karimi, S., Bahari, A., Nourian, A. et al. Neospora caninum and Toxoplasma gondii infections in one-humped camels (Camelus dromedarius) in central desert of Iran. Parasitol Res 122, 847–852 (2023). https://doi.org/10.1007/s00436-023-07783-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07783-w