Abstract
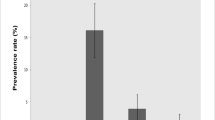
Animal trypanosomosis is an important endemic and wasting disease in sub-Saharan Africa. Its control relies on chemotherapy, and resistance to trypanocides has been widely reported. The pathogenicity of drug-resistant canine trypanosomes is not clear with scanty information available. Thus, this study assessed the comparative pathogenicity of drug-resistant and drug-sensitive Trypanosoma brucei and Trypanosoma congolense infections in dogs. Twenty Nigerian local dogs were used and were randomly assigned into five groups (A–E) of four dogs each. Group A served as the uninfected-control group, while groups B and C were infected with 106 drug-sensitive T. congolense and T. brucei. Groups D and E were infected with 106 multidrug-resistant T. congolense and T. brucei, respectively. The pre-patent period (PPP), clinical signs, level of parasitaemia (LOP), rectal temperature, body weight, packed cell volume (PCV), red blood cell count (RBC), haemoglobin concentration (HbC), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), total leucocyte count (TLC) and survivability were assessed. Groups D and E had longer (p < 0.05) mean PPP than groups B and C. Also, group E dogs had lower (p < 0.05) mean LOP, longer (p < 0.05) mean survivability, and higher (p < 0.05) mean body weight, PCV, HbC and RBC than group C dogs. The clinical signs were very severe in group C dogs, compared to group E dogs. However, these parameters did not differ statistically between groups B and D. Thus, multidrug-resistant T. brucei was of lower pathogenicity than drug-sensitive T. brucei, while multidrug-resistant and drug-sensitive T. congolense had comparable pathogenicity following infection in dogs.










Similar content being viewed by others
Data availability
All additional data associated with this study can be obtained from the corresponding author on reasonable request.
References
Anene BM, Chukwu CC, Anika SM (1999) Sensitivity to diminazene aceturate and isometamidium chloride of trypanosomes isolated from dogs in Nsukka area, Nigeria. Revue Élev Méd Vét Pays Trop 52(2):129–131
Bengaly Z, Sidibe I, Ganaba R, Desquesnes M, Boly H, Sawadogo L (2002) Comparative pathogenicity of three genetically distinct types of Trypanosoma congolense in cattle: clinical observations and haematological changes. Vet Parasitol 108:1–19. https://doi.org/10.1016/S0304-4017(02)00164-4
Burudi EM, Peregrine AS, Mejia PO, Mbiuki SM, Murphy NB (1994) Response of diminazine aceturate resistant and diminazine aceturate susceptible Trypanosoma congolense to treatment with Diminazine when occurring together as a mixed infection. Ann Tropl Med Parasitol 88(6):595–606. https://doi.org/10.1080/00034983.1994.11812910
Coleman PG, Eisler MC, McDermott JJ, Murphy NB, Majiwa PAO, Peregrine AS (2000) Relative fitness in laboratory mice of drug-resistant and drug-sensitive field isolates of Trypanosoma congolense. Australasian e-Library of Veterinary & Animal Science. https://www.sciquest.org.nz/browse/publications/article/71162. Accessed 15 Feb 2022
Coles EH (1986) Veterinary clinical pathology, 4th edn. WB Saunders, Philadelphia
Dacie J, Bain B, Imelda B, Lewis M (2001) Practical haematology, 9th edn. Churchill Livingstone, London, pp 34–35
Delespaux V, Geysen D, Van den Bossche P, Geerts S (2008) Molecular tools for the rapid detection of drug resistance in animal trypanosomes. Trends Parasitol 24:236–242. https://doi.org/10.1016/j.pt.2008.02.006
Egbe-Nwiyi TN, Igbokwe IO, Onyeyili PA (2005) Diminzene aceturate resistance on the virulence of Trypanosoma brucei for rats. J Comp Path 133:286–288. https://doi.org/10.1016/j.jcpa.2005.05.002
Ezeokonkwo RC, Ezeh IO, Onukwu JI, Obi PO, Onyenwe IW, Agu WE (2010) Comparative haematological study of single and mixed infections of mongrel dogs Trypanosoma congolense and Trypanosoma brucei brucei. Vet Parasitol 173:48–54. https://doi.org/10.1016/j.vetpar.2010.06.020
Geerts S, Holmes PH, Diall O, Eisler MC (2001) African bovine trypanosomiasis. the problem of drug resistance. Trends Parasitol 17:25–28. https://doi.org/10.1016/S1471-4922(00)01827-4
Geerts S, Holmes PH (1998) Drug management and parasite resistance in bovine trypanosomosis in Africa. Program against African Trypanosomes (PAAT). Technical and scientific series 1. Food Agric Org U N (FAO) Rome, 25:1–3.
Giha HA, Elbashir MI, A-Elbasit IE, A-Elgadir TME, El-Ghazali GE, Mackinnon MJ, Babiker HA (2006) Drug resistance–virulence relationship in Plasmodium falciparum causing severe malaria in an area of seasonal and unstable transmission. Acta Trop 97:181–187. https://doi.org/10.1016/j.actatropica.2005.10.004
Giordani F, Morrison LJ, Rowan TG, De Koning HP, Barrett MP (2016) The animal trypanosomiases and their chemotherapy: a review. Parasitol 143(14):1862–1889. https://doi.org/10.1017/S0031182016001268
Herbert WJ, Lumsden WH (1976) Trypanosoma brucei: a rapid “Matching” method for estimating the host’s parasitaemia. Exp Parasitol 40:427–431. https://doi.org/10.1016/0014-4894(76)90110-7
Hugo KM (2011) Molecular characterization and pathogenicity of melarsoprol resistant and sensitive Trypanosoma brucei rhodesiense isolates from Uganda. Dissertation, Egerton University
Ihedioha JI, Onuma CJ, Okorie-Kanu CO, Ihedioha TE (2010) Changes in the pathogenicity of relapsed diminazene aceturate (DA)-resistant Trypanosoma brucei brucei as the trypanosomes are transmitted from DA-treated hosts to another set of animals. Comp Clin Pathol 19:481–486. https://doi.org/10.1007/s00580-009-0893-9
Kaboré J, Camara O, Koffi M, Sanou D, Ilboudo H, Sakandé H, Camara M, De Meeûs T, Ravel S, Belem AMG, MacLeod A, Bucheton B, Jamonneau V, Thévenon S (2018) Differences in pathogenicity and virulence of Trypanosoma brucei gambiense field isolates in experimentally infected Balb/C mice. Infect Genet Evol 63:269–276. https://doi.org/10.1016/j.meegid.2018.05.018
Levin BR, Perrot V, Walker N (2000) Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria. Genet 154:985–997. https://doi.org/10.1093/genetics/154.3.985
MacGregor P, Szöoõr B, Savill NJ, Matthews KR (2012) Trypanosomal immune evasion, chronicity and transmission: an elegant balancing act. Nat Rev Microbiol 10(6):431–438. https://doi.org/10.1038/nrmicro2779
Masumu J, Marcotty T, Geysen D, Geerts S, Vercruysse J, Dorny P, den Bossche PV (2006) Comparison of the virulence of Trypanosoma congolense strains isolated from cattle in a trypanosomiasis endemic area of eastern Zambia. Int J Parasitol 36:497–501. https://doi.org/10.1016/j.ijpara.2006.01.003
Mbaya A, Kumshe H, Nwosu CO (2012) The mechanisms of anaemia in trypanosomosis: a review. In: Silverberg D (ed) Anaemia, InTechopen. https://doi.org/10.5772/29530
McDermott J, Woitag T, Sidibe I, Bauer B, Diarra B, Oue´draogo D, Kamuanga M, Peregrine A, Eisler M, Zessin K-H, Mehlitz D, Clausen P-H (2003) Field studies of drug-resistant cattle trypanosomes in Ke´ne´dougou Province, Burkina Faso. Acta Trop 86:93–103. https://doi.org/10.1016/S0001-706X(03)00019-6
Motloang MY, Masumu J, Mans BJ, Latif AA (2014) Virulence of Trypanosoma congolense strains isolated from cattle and African buffaloes (Synceru scaffer) in KwaZulu-Natal. South Africa Onderstepoort J Vet Res 81:679. https://doi.org/10.4102/ojvr.v81i1.679
Mulandane FC, Fafetine J, Van Den Abbeele J, Clausen P-H, Hoppenheit A, Cecchi G, Oosthuizen M, Delespaux V, Neves L (2017) Resistance to trypanocidal drugs in cattle populations of Zambezia Province, Mozambique. Parasitol Res https://doi.org/10.1007/s00436-017-5718-1
Murray M (1979) The anaemia of bovine African trypanosomiasis: an overview. In: Losos G, Chouinard A (eds) Pathogenicity of Trypanosomes. IDRC, Ottawa, pp 121–127
Mutangala NW (2011) Transmisibility and virulence study of drug sensitive and resistant Trypanosoma congolense in mice. Dissertation, Egerton University
Ngumbi A, Mnyone L (2020) Pathogenicity of trypanosomes in relation to 1 drug sensitivity: comparative studies between a drug-sensitive and drug-resistant Trypanosoma congolense strain in murine-and bovine model. Preprint, https://doi.org/10.21203/rs.3.rs-17414/v1
Obi CF, Obidike IR, Ezeh IO, Omoja VU, Iheagwam CN, Idika IK, Ezeokonkwo RC (2013) Effects of Trypanosoma brucei infection and diminazene aceturate therapy on testicular morphology and function of Nigerian local dogs. Vet Parasitol 196(3):283–288. https://doi.org/10.1016/j.vetpar.2013.03.023
Obi CF, Nzeakor TA, Okpala MI, Ezeh IO, Nwobi LG, Omeje MO, Ezeokonkwo RC (2019) Evaluation of antitrypanosomal activity of Pterocarpus santalinoides L’H’erit ex DC hydroethanol leaf extract in rats experimentally infected with Trypanosoma brucei. J Ethnopharmacol 243:112085. https://doi.org/10.1016/j.jep.2019.112085
Obi CF, Ezeh IO, Okpala MI, Agina A, Umeakuana PU, Essuman GAA, Gwira TM, Ezeokonkwo RC (2022a) Prevalence and molecular identification of trypanosomes in dogs in Enugu North Senatorial Zone. South East Nigeria. Acta Parasitol 67(1):391–402. https://doi.org/10.1007/s11686-021-00475-4
Obi CF, Okpala MI, Ezeh IO, Onyeabo A, Ezeokonkwo RC (2022b) Drug-resistant trypanosome isolates populations in dogs in Enugu North Senatorial Zone. Southeastern Nigeria. Parasitol Res 121(1):423–431. https://doi.org/10.1007/s00436-021-07362-x
Odette AE (2011) Transmissibility and virulence of drug sensitive and resistant Trypanosoma brucei rhodesiense isolates from Kenya. Dissertation, Egerton University
Otavio LFJ, Sampaio PH, Machado RZ, André MR, Marques LC, Cadioli FA (2016) Evaluation of clinical signs, parasitemia, hematologic and biochemical changes in cattle experimentally infected with Trypanosoma vivax. Rev Bras Parasitol Vet 25(1). https://doi.org/10.1590/S1984-29612016013
Perrone T, Aso PM, Mijares A, Holzmuller P, Gonzatti M, Parra N (2018) Comparison of infectivity and virulence of clones of Trypanosoma evansi and Trypanosoma equiperdum Venezuelan strains in mice. Vet Parasitol 253:60–64. https://doi.org/10.1016/j.vetpar.2018.02.024
Schneider P, Bell AS, Sim DG, O’Donnell AJ, Blanford S, Paaijmans KP, Read AF, Reece SE (2012) Virulence, drug sensitivity and transmission success in the rodent malaria Plasmodium Chabaudi. Proc Biol Sci 279:4677–4685. https://doi.org/10.1098/rspb.2012.1792
Silva RAMS, Arosemena NAE, Herrera HM, Sahib CA, Ferreira MSJ (1995) Outbreak of trypanosomosis due to Trypanosoma evansi in horses of pantanal Mato-grossense, Brazil. Vet Parasitol 60:167–171. https://doi.org/10.1016/0304-4017(94)00757-4
Taylor K, Authie EML (2004) Pathogenesis of animal trypanosomosis. In: Maudin I, Holmes PH, Miles MA (eds) The trypanosomiasis. CAB International, UK, pp 331–353
Tekle T, Terefe G, Cherenet T, Ashenafi H, Akoda KG, Teko-Agbo A, van den Abbeele J, Gari G, Clausen P-H, Hoppenheit A, Mattioli RC, Peter R, Marcotty T, Cecchi G, Delespaux V (2018) Aberrant use and poor quality of trypanocides: a risk for drug resistance in south western Ethiopia. BMC Vet Res 14:4. https://doi.org/10.1186/s12917-017-1327-6
Tesfaye B, Getachew A, Hagos A, Yacob HT (2012) Comparative study on the pathogenic effects of Diminazine aceturate sensitive and resistant isolates of Trypanosoma congolense in goats. Ethiop Vet J 16(1):59–69. https://doi.org/10.4314/evj.v16i1.5
Thuita JK, Kagira JM, Mwangangi D, Matovu E, Turner CM, Masiga D (2008) Trypanosoma brucei rhodesiense transmitted by a single tsetse fly bite in vervet monkeys as a model of human African trypanosomiasis. PLoS Negl Trop Dis 2(5):e238. https://doi.org/10.1371/journal.pntd.0000238
Funding
This study was supported by the Nigerian Tertiary Education Trust Fund (TETFUND) through the TETFUND Institution Based Research (IBR) intervention (TETFUND/ DESS/UNI/NSUKKA/2018/RP/VOL.1).
Author information
Authors and Affiliations
Contributions
RCE, IOE and CFO contributed to the study design and conception; RCE secured funding; CFO, MIO, DCA, AO and IOE performed the experiment; CFO conducted the statistical analysis of the data and wrote the first draft of the manuscript; RCE, IOE and CFO reviewed and edited the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Valid ethical clearance (FVM-UNN-IACUC-2019–0922) was gotten from the University of Nigeria Faculty of Veterinary Medicine Institutional Animal Care and Use Committee. More so, the University of Nigeria, national and international guidelines for the ethical care and use of animals were fully observed.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Obi, C.F., Okpala, M.I., Anyogu, D.C. et al. Comparative pathogenicity of drug-resistant and drug-sensitive Trypanosoma brucei and Trypanosoma congolense infections in Nigerian local dogs. Parasitol Res 122, 49–60 (2023). https://doi.org/10.1007/s00436-022-07688-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07688-0