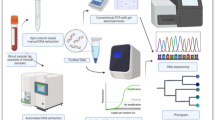
Abstract
Human babesiosis is caused by Babesia duncani that is transmitted through tick bites, blood transfusions, and transplacental transmission. Despite its health burden, diagnostic assays for this pathogen are either unsuitable for clinical applications or have a low detection efficiency; therefore, it remains undetected during transfusion and utilization of blood and blood-component transfusions. This study used a molecular approach via nested quantitative polymerase chain reaction (qPCR) by designing primers and probes corresponding to the variable regions of B. duncani 18S rRNA gene to specifically detect B. duncani DNA in experimentally infected LVG Golden Syrian hamster (n = 70) and human (n = 492; tick bite patients from Gansu Province, China) blood samples. Moreover, comparative analyses of this technique with previously reported nested PCR and microscopy were conducted. The newly optimized diagnostic technique exhibited no cross-reactivity with genomic DNA or plasmids containing the 18S rRNA gene of other zoonotically important Babesia spp., including B. microti, B. divergens, B. crassa, and B. motasi Hebei. The detection limit of nested qPCR was approximately one plasmid copy in 20 μL or one infected red blood cell in 200 μL whole blood. The specificity and sensitivity of the method were 100% and 98.6%, respectively. Comparative analyses revealed that nested qPCR detected B. duncani had relatively higher efficacy and specificity than microscopic examination and nested PCR. The 492 human blood samples were negative for B. duncani infection. Thus, the present study provides an improved diagnostic assay for the efficient and effective detection and analysis of B. duncani infections and its prevalence in infection-prone areas.



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the published article.
Abbreviations
- ATCC:
-
American Type Culture Collection
- PCR:
-
Polymerase chain reaction
- qPCR:
-
Real-time PCR
- ME:
-
Microscopic examination
- VVBD:
-
Vectors and Vector-Borne Diseases Laboratory
- LVRI:
-
Lanzhou Veterinary Research Institute
- TTB:
-
Transfusion transmitted babesiosis
- iRBCs:
-
Infected red blood cells
- PPE:
-
Percentage of parasitized erythrocytes
- IFA:
-
Immunofluorescence
- FISH:
-
Fluorescence in situ hybridization
References
Abraham A, Brasov I, Thekkiniath J, Kilian N, Lawres L, Gao R, DeBus K, He L, Yu X, Zhu G, Graham MM, Liu X, Molestina R, Ben Mamoun C (2018) Establishment of a continuous in vitro culture of Babesia duncani in human erythrocytes reveals unusually high tolerance to recommended therapies. J Biol Chem 293:19974–19981. https://doi.org/10.1074/jbc.AC118.005771
Bajer A, Dwuznik-Szarek D (2021) The specificity of Babesia-tick vector interactions: recent advances and pitfalls in molecular and field studies. Parasit Vectors 14:507. https://doi.org/10.1186/s13071-021-05019-3
Baltrunaite L, Kitryte N, Krizanauskiene A (2020) Blood parasites (Babesia, Hepatozoon and Trypanosoma) of rodents, Lithuania: part I. Molecular and traditional microscopy approach. Parasitol Res 119:687–694. https://doi.org/10.1007/s00436-019-06577-3
Busch MP, Bloch EM, Kleinman S (2019) Prevention of transfusion-transmitted infections. Blood 133:1854–1864. https://doi.org/10.1182/blood-2018-11-833996
Caccio C, Camma C, Onuma M, Severini C (2000) The beta-tubulin gene of Babesia and Theileria parasites is an informative marker for species discrimination. Int J Parasitol 30:1181–1185. https://doi.org/10.1016/S0020-7519(00)00105-3
Chen Z, Li H, Gao X, Bian A, Yan H, Kong D, Liu X (2019) Human babesiosis in China: a systematic review. Parasitol Res 118:1103–1112. https://doi.org/10.1007/s00436-019-06250-9
Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, Quick R, Telford SR 3rd, Herwaldt BL (2006) Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol 36:779–789. https://doi.org/10.1016/j.ijpara.2006.03.008
Fang LQ, Liu K, Li XL, Liang S, Yang Y, Yao HW, Sun RX, Sun Y, Chen WJ, Zuo SQ, Ma MJ, Li H, Jiang JF, Liu W, Yang XF, Gray GC, Krause PJ, Cao WC (2015) Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect Dis 15:1467–1479. https://doi.org/10.1016/S1473-3099(15)00177-2
Flaherty BR, Talundzic E, Barratt J, Kines KJ, Olsen C, Lane M, Sheth M, Bradbury RS (2018) Restriction enzyme digestion of host DNA enhances universal detection of parasitic pathogens in blood via targeted amplicon deep sequencing. Microbiome 6:164. https://doi.org/10.1186/s40168-018-0540-2
Flaherty BR, Barratt J, Lane M, Talundzic E, Bradbury RS (2021) Sensitive universal detection of blood parasites by selective pathogen-DNA enrichment and deep amplicon sequencing. Microbiome 9:1. https://doi.org/10.1186/s40168-020-00939-1
Fox LM, Wingerter S, Ahmed A, Arnold A, Chou J, Rhein L, Levy O (2006) Neonatal babesiosis: case report and review of the literature. Pediatr Infect Dis J 25:169–173. https://doi.org/10.1097/01.inf.0000195438.09628.b0
Jia N, Zheng YC, Jiang JF et al (2018) Human babesiosis caused by a Babesia crassa-like pathogen: a case series. Clin Infect Dis 67:1110–1119. https://doi.org/10.1093/cid/ciy212
Joseph JT, Purtill K, Wong SJ, Munoz J, Teal A, Madison-Antenucci S, Horowitz HW, Aguero-Rosenfeld ME, Moore JM, Abramowsky C, Wormser GP (2012) Vertical transmission of Babesia microti, United States. Emerg Infect Dis 18:1318–1321. https://doi.org/10.3201/eid1808.110988
Karshima SN, Karshima MN, Ahmed MI (2022) Infection rates, species diversity, and distribution of zoonotic Babesia parasites in ticks: a global systematic review and meta-analysis. Parasitol Res 121:311–334. https://doi.org/10.1007/s00436-021-07359-6
Krause PJ (2019) Human babesiosis. Int J Parasitol 49:165–174. https://doi.org/10.1016/j.ijpara.2018.11.007
Krause PJ, Lepore T, Sikand VK, Gadbaw J Jr, Burke G, Telford SR 3rd, Brassard P, Pearl D, Azlanzadeh J, Christianson D, McGrath D, Spielman A (2000) Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 343:1454–1458. https://doi.org/10.1056/NEJM200011163432004
Krause PJ, Auwaerter PG, Bannuru RR, Branda JA, Falck-Ytter YT, Lantos PM, Lavergne V, Meissner HC, Osani MC, Rips JG, Sood SK, Vannier E, Vaysbrot EE, Wormser GP (2021) Clinical practice guidelines by the infectious diseases society of America (IDSA): 2020 guideline on diagnosis and management of babesiosis. Clin Infect Dis 72:e49–e64. https://doi.org/10.1093/cid/ciaa1216
Leiby DA (2011a) Transfusion-associated babesiosis: shouldn’t we be ticked off? Ann Intern Med 155:556–557. https://doi.org/10.7326/0003-4819-155-8-201110180-00363
Leiby DA (2011b) Transfusion-transmitted Babesia spp.: bull’s-eye on Babesia microti. Clin Microbiol Rev 24:14–28. https://doi.org/10.1128/CMR.00022-10
Lobo CA, Singh M, Rodriguez M (2020) Human babesiosis: recent advances and future challenges. Curr Opin Hematol 27:399–405. https://doi.org/10.1097/MOH.0000000000000606
Man SQ, Qiao K, Cui J, Feng M, Fu YF, Cheng XJ (2016) A case of human infection with a novel Babesia species in China. Infect Dis Poverty 5:28. https://doi.org/10.1186/s40249-016-0121-1
Martinez-Garcia G, Santamaria-Espinosa RM, Lira-Amaya JJ, Figueroa JV (2021) Challenges in tick-borne pathogen detection: the case for Babesia spp. identification in the tick vector. Pathogens 10:92. https://doi.org/10.3390/pathogens10020092
O’Connor KE, Kjemtrup AM, Conrad PA, Swei A (2018) An improved PCR protocol for detection of Babesia duncani in wildlife and vector samples. J Parasitol 104:429–432. https://doi.org/10.1645/17-155
Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA (2012) Babesia: a world emerging. Infect Genet Evol 12:1788–1809. https://doi.org/10.1016/j.meegid.2012.07.004
Scott JD, Scott CM (2018) Human babesiosis caused by Babesia duncani has widespread distribution across Canada. Healthcare (basel) 6:49. https://doi.org/10.3390/healthcare6020049
Shah JS, Caoili E, Patton MF, Tamhankar S, Myint MM, Poruri A, Mark O, Horowitz RI, Ashbaugh AD, Ramasamy R (2020a) Combined immunofluorescence (IFA) and fluorescence in situ hybridization (FISH) assays for diagnosing babesiosis in patients from the USA. Eur Aust Diagn (basel) 10:761. https://doi.org/10.3390/diagnostics10100761
Shah JS, Mark O, Caoili E, Poruri A, Horowitz RI, Ashbaugh AD, Ramasamy R (2020b) A fluorescence in situ hybridization (FISH) test for diagnosing babesiosis. Diagn (basel) 10:377. https://doi.org/10.3390/diagnostics10060377
Simon MS, Westblade LF, Dziedziech A, Visone JE, Furman RR, Jenkins SG, Schuetz AN, Kirkman LA (2017) Clinical and molecular evidence of atovaquone and azithromycin resistance in relapsed Babesia microti infection associated with rituximab and chronic lymphocytic leukemia. Clin Infect Dis 65:1222–1225. https://doi.org/10.1093/cid/cix477
Smith RP, Hunfeld KP, Krause PJ (2020) Management strategies for human babesiosis. Expert Rev Anti Infect Ther 18:625–636. https://doi.org/10.1080/14787210.2020.1752193
Stanley J, Stramer SL, Erickson Y, Cruz J, Gorlin J, Janzen M, Rossmann SN, Straus T, Albrecht P, Pate LL, Galel SA, cobas Babesia IND study group, (2021) Detection of Babesia RNA and DNA in whole blood samples from US blood donations. Transfusion 61:2969–2980. https://doi.org/10.1111/trf.16617
Thomford JW, Conrad PA, Telford SR 3rd, Mathiesen D, Bowman BH, Spielman A, Eberhard ML, Herwaldt BL, Quick RE, Persing DH (1994) Cultivation and phylogenetic characterization of a newly recognized human pathogenic protozoan. J Infect Dis 169:1050–1056. https://doi.org/10.1093/infdis/169.5.1050
Tonnetti L, Dodd RY, Foster G, Stramer SL (2022) Babesia blood testing: the first-year experience. Transfusion 62:135–142. https://doi.org/10.1111/trf.16718
Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ (2015) Babesiosis. Infect Dis Clin North Am 29:357–370. https://doi.org/10.1016/j.idc.2015.02.008
Wagner SJ, Leiby DA, Roback JD (2019) Existing and emerging blood-borne pathogens impact on the safety of blood transfusion for the hematology/oncology patient. Hematol Oncol Clin North Am 33:739–748. https://doi.org/10.1016/j.hoc.2019.05.002
Wang J, Liu J, Yang J, Wang X, Li Z, Jianlin X, Li X, Xiang Q, Li Y, Liu Z, Luo J, Guan G, Yin H (2019) The first molecular detection and genetic diversity of Babesia caballi and Theileria equi in horses of Gansu province, China. Ticks Tick Borne Dis 10:528–532. https://doi.org/10.1016/j.ttbdis.2019.01.003
Wang J, Gao S, Zhang S, He X, Liu J, Liu A, Li Y, Liu G, Luo J, Guan G, Yin H (2020) Rapid detection of Babesia motasi responsible for human babesiosis by cross-priming amplification combined with a vertical flow. Parasit Vectors 13:377. https://doi.org/10.1186/s13071-020-04246-4
Wilson M, Glaser KC, Adams-Fish D, Boley M, Mayda M, Molestina RE (2015) Development of droplet digital PCR for the detection of Babesia microti and Babesia duncani. Exp Parasitol 149:24–31. https://doi.org/10.1016/j.exppara.2014.12.003
Wilson MR, Sample HA, Zorn KC et al (2019) Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med 380:2327–2340. https://doi.org/10.1056/NEJMoa1803396
Wozniak EJ, Lowenstine LJ, Hemmer R, Robinson T, Conrad PA (1996) Comparative pathogenesis of human WA1 and Babesia microti isolates in a Syrian hamster model. Lab Anim Sci 46:507–515
Young KM, Corrin T, Wilhelm B, Uhland C, Greig J, Mascarenhas M, Waddell LA (2019) Zoonotic Babesia: a scoping review of the global evidence. PLoS ONE 14:e0226781. https://doi.org/10.1371/journal.pone.0226781
Funding
This study was financially supported by the National Key Research and Development Program of China (2017YFD0501200), NSFC (No. 1972706), NSFC (No. 31972701), and Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Disease and Zoonoses.
Author information
Authors and Affiliations
Contributions
Wang Yanbo wrote the main manuscript text, and Zhang Shangdi and Wang Xiaorong collected the specimens. Wang Jinming and Muhammad Rashid revised the article, and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The collection and manipulation of hamster blood samples were approved by the Animal Ethics Committee of the Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. All sampling procedures were handled in accordance with the Animal Ethics Procedures and Guidelines of the People’s Republic of China (Permit No. LVRIAEC-2020–001). The study of clinical specimens was approved by the Ethics Committee of The Second Hospital of Lanzhou University (reference 2018A-046). All the procedures were conducted according to the Ethical Procedures and Guidelines of the People’s Republic of China.
Consent to participate
All authors consent to be participated.
Consent for publication
All authors consent to be published.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Zhang, S., Wang, J. et al. Nested qPCR assay to detect Babesia duncani infection in hamsters and humans. Parasitol Res 121, 3603–3610 (2022). https://doi.org/10.1007/s00436-022-07685-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07685-3