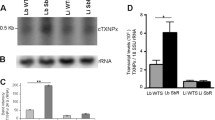
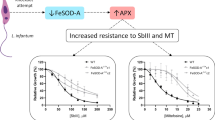
Abstract
Tyrosine aminotransferase is a well-characterized enzyme in the Leishmania parasite, but the role of TAT in the parasite functioning remains largely unknown. In this study, we attempt to gain a better understanding of the enzyme’s role in the parasite by gene knockout and overexpression of the TAT gene. The overexpression of TAT protein was well tolerated by the parasites in two independent repeats. Single knockout of TAT gene by homologous recombination, LdTAT+/- displayed distinct retardation in the proliferation rates and entered the death phase immediately. Morphology of LdTAT+/- parasites had important structural defects as they rounded up with elongated flagella. Gene regulation studies suggested the upregulation of key apoptotic and redox metabolism genes in LdTAT+/-. Moreover, LdTAT+/- cells accumulated higher ROS, thiols, intracellular Ca2+ concentrations, and mitochondrial membrane depolarization signifying the onset of apoptosis. Tocopherol levels were reduced by 50% in LdTAT+/- suggesting the involvement of TAT in tocopherol biosynthesis in the parasite. Overall, our results provide the first evidence that gene knockout of TAT results in apoptosis and that TAT is required for the survival and viability of Leishmania donovani.











Similar content being viewed by others
Availability of Data and Material
All data and materials described in this study are available with the corresponding author upon request.
References
Amin AS (2001) Colorimetric determination of tocopheryl acetate (vitamin E) in pure form and in multi-vitamin capsules. Eur J Pharm Biopharm 51(3):267–272
Balanco JMF, Sussmann RA, Verdaguer IB, Gabriel HB, Kimura EA, Katzin AM (2019) Tocopherol biosynthesis in Leishmania (L.) amazonensis promastigotes. FEBS Open Biol 9(4):743–754
Beverley SM, Ellenberger TE, Cordingley JS (1986) Primary structure of the gene encoding the bifunctional dihydrofolate reductase-thymidylate synthase of Leishmania major. Proc Natl Acad Sci 83(8):2584–2588
Blankenfeldt W, Nowicki C, Montemartini-Kalisz M, Kalisz HM, Hecht HJ (1999) Crystal structure of Trypanosoma cruzi tyrosine aminotransferase: Substrate specificity is influenced by cofactor binding mode. Protein Sci 8:2406–2417
Cameron P, McGachy A, Anderson M, Paul A, Coombs GH, Mottram JC, Alexander J, Plevin R (2004) Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-κB signaling pathway. J Immunol 173(5):3297–3304
Cazzulo Franke MC, Vernal J, Cazzulo JJ, Nowicki C (1999) The NAD-linked aromatic α-hydroxy acid dehydrogenase from Trypanosoma cruzi: a new member of the cytosolic malate dehydrogenases group without malate dehydrogenase activity. Eur J Biochem 266(3):903–910
Cristina Nowicki MM, Duschak V, Santome JA, Cazzulo JJ (1992) Presence and subcellular localization of tyrosine aminotransferase and p-hydroxyphenyllactate dehydrogenase in epimastigotes of Trypanosoma cruzi. FEMS Microbiol Lett 92:119–124
Croft SL, Sundar S, Fairlamb AH (2006) Drug resistance in leishmaniasis. Clin Microbiol Rev 19(1):111–126
Denise H, Poot J, Jiménez M, Ambit A, Herrmann DC, Vermeulen AN, Coombs GH, Mottram JC (2006) Studies on the CPA cysteine peptidase in the Leishmania infantum genome strain JPCM5. BMC Mol Biol 7(1):1–13
Efstathiou A, Smirlis D (2021) Leishmania protein kinases: important regulators of the parasite life cycle and molecular targets for treating leishmaniasis. Microorganisms 9(4):691
Freedman DJ, Beverley SM (1993) Two more independent selectable markers for stable transfection of Leishmania. Mol Biochem Parasitol 62(1):37–44
Gee KR, Brown K, Chen WU, Bishop-Stewart J, Gray D, Johnson I (2000) Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium 27(2):97–106
Ha DS, Schwarz JK, Turco SJ, Beverley SM (1996) Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol 77(1):57–64
Kathuria M, Bhattacharjee A, Sashidhara KV, Singh SP, Mitra K (2014) Induction of mitochondrial dysfunction and oxidative stress in Leishmania donovani by orally active clerodane diterpene. Antimicrob Agents Chemother 58(10):5916–5928
Krassner SM, Flory B (1971) Essential amino acids in the culture of Leishmania tarentolae. J Parasitol 1971:917–920
Krauth-Siegel RL, Comini MA (2008) Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim et Biophysica Acta (BBA)-General Subjects 1780(11):1236–1248
Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke E, Blagosklonny M, El-Deiry W, Golstein P, Green D (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16(1):3–11
Leroux A, Fleming-Canepa X, Aranda A, Maugeri D, Cazzulo JJ, Sanchez MA, Nowicki C (2006) Functional characterization and subcellular localization of the three malate dehydrogenase isozymes in Leishmania spp. Mol Biochem Parasitol 149(1):74–85
Marciano D, Maugeri DA, Cazzulo JJ, Nowicki C (2009) Functional characterization of stage-specific aminotransferases from trypanosomatids. Mol Biochem Parasitol 166(2):172–182
Marisa Montemartini JAS, Cazzulo JJ, Nowicki C (1993) Purification and partial structural and kinetic characterization of tyrosine aminotransferase from epimastigotes of Trypanosoma cruzi. Biochem J 292:901–906
Marisa Montemartini JB, Bontempi E, Zelada C, Andres JAS, Ruiz M, Cazzulo JJ, Nowicki C (1995) A recombinant tyrosine aminotransferase from Trypanosoma cruzi has both tyrosine aminotransferase and alanine aminotransferase activities. FEMS Microbiol Lett 133:17–20
Mehere P, Han Q, Lemkul JA, Vavricka CJ, Robinson H, Bevan DR, Li J (2010) Tyrosine aminotransferase: biochemical and structural properties and molecular dynamics simulations. Protein Cell 1(11):1023–1032
Mishra J, Saxena A, Singh S (2007) Chemotherapy of leishmaniasis: past, present and future. Curr Med Chem 14(10):1153–1169
Montemartini M, Santomé J, Cazzulo JJ, Nowicki C (1994) Production of aromatic α-hydroxyacids by epimastigotes of Trypanosoma cruzi, and its possible role in NADH reoxidation. FEMS Microbiol Lett 118(1-2):89–92
Moreno MA, Alonso A, Alcolea PJ, Abramov A, de Lacoba MG, Abendroth J, Zhang S, Edwards T, Lorimer D, Myler PJ, Larraga V (2014a) Tyrosine aminotransferase from Leishmania infantum: a new drug target candidate. Int J Parasitol Drugs Drug Resist 4(3):347–354
Moreno MA, Abramov A, Abendroth J, Alonso A, Zhang S, Alcolea PJ, Edwards T, Lorimer D, Myler PJ, Larraga V (2014b) Structure of tyrosine aminotransferase from Leishmania infantum. Acta Crystallogr F Struct Biol Commun 70(Pt 5):583–587
Mottram JC, Souza AE, Hutchison JE, Carter R, Frame MJ, Coombs GH (1996) Evidence from disruption of the lmcpb gene array of Leishmania mexicana that cysteine proteinases are virulence factors. Proc Natl Acad Sci 93(12):6008–6013
Myler P, Fasel N (2008) Leishmania: after the genome. Caister Academic Press, Norfolk
Palmer MS, Dryden AJ, Hughes JT, Collinge J (1991) Homozygous prion protein genotype predisposes to sporadic Creutzfeld-Jakob disease. Nature 352(34):342
Rai S, Goel SK, Nath Dwivedi U, Sundar S, Goyal N (2013) Role of efflux pumps and intracellular thiols in natural antimony resistant isolates of Leishmania donovani. PLoS One 8(9):e74862
Raj S, Saha G, Sasidharan S, Dubey VK, Saudagar P (2019) Biochemical characterization and chemical validation of Leishmania MAP Kinase-3 as a potential drug target. Sci Rep 9(1):1–11
Rege AA (1987) Purification and characterization of a tyrosine aminotransferase from Crithidia fasciculata. Mol Biochem Parasitol 25:1–9
Sasidharan S, Saudagar P (2019) Biochemical and structural characterization of tyrosine aminotransferase suggests broad substrate specificity and a two-state folding mechanism in Leishmania donovani. FEBS Open Biol 9(10):1769–1783
Sasidharan S, Saudagar P (2020a) Concerted motion of structure and active site charge is required for tyrosine aminotransferase activity in Leishmania parasite. Spectrochim Acta A Mol Biomol Spectrosc 232:118133
Sasidharan S, Saudagar P (2020b) Flavones reversibly inhibit Leishmania donovani tyrosine aminotransferase by binding to the catalytic pocket: an integrated in silico-in vitro approach. Int J Biol Macromol 164:2987–3004
Sasidharan S, Saudagar P (2020c) Mapping N-and C-terminals of Leishmania donovani tyrosine aminotransferase by gene truncation strategy: a functional study using in vitro and in silico approaches. Sci Rep 10(1):1–15
Sasidharan S, Saudagar P (2021) Leishmaniasis: where are we and where are we heading? Parasitol Res 120(5):1541–1554
Sasidharan S, Tripathi T, Saudagar P (2021) Critical insight into plausible acquired tocopherol pathway in neglected human trypanosomatids. ACS Omega 6:31396
Shadab M, Jha B, Asad M, Deepthi M, Kamran M, Ali N (2017) Apoptosis-like cell death in Leishmania donovani treated with KalsomeTM10, a new liposomal amphotericin B. PLoS One 12(2):e0171306
Sivandzade F, Bhalerao A, Cucullo L (2019) Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio-protocol 9(1):e3128–e3128
Sivaraman S, Kirsch JF (2006) The narrow substrate specificity of human tyrosine aminotransferase--the enzyme deficient in tyrosinemia type II. FEBS J 273(9):1920–1929
Stibbs H, Seed J (1973) Chromatographic evidence for the synthesis of possible sleep-mediators inTrypanosoma brucei gambiense. Experientia 29(12):1563–1565
Tanz SK, Castleden I, Small ID, Millar AH (2013) Fluorescent protein tagging as a tool to define the subcellular distribution of proteins in plants. Front Plant Sci 4:214
World Health Organization (2010) Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases (No. WHO/HTM/NTD/2010.1). World Health Organization. Vancouver
Acknowledgments
The author SS acknowledges research fellowship from NIT Warangal. Research funding by the Science and Engineering Research Board, DST, Government of India (Grant Number: EEQ/2018/000484) is acknowledged.
Author information
Authors and Affiliations
Contributions
Santanu Sasidharan: Conceptualization, methodology, inspection; visualization, and writing—original draft preparation. Prakash Saudagar: Supervision; conceptualization; writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Dorien Mabille
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 370 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sasidharan, S., Saudagar, P. Knockout of Tyrosine Aminotransferase Gene by Homologous Recombination Arrests Growth and Disrupts Redox Homeostasis in Leishmania Parasite. Parasitol Res 121, 3229–3241 (2022). https://doi.org/10.1007/s00436-022-07642-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07642-0