Abstract
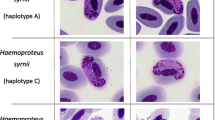
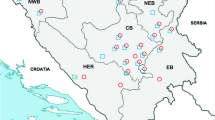
Espinilho savanna (“seasonal steppe savanna”) is a unique vegetation formation of the Pampas biome that is found near the tri-border of Brazil, Uruguay, and Argentina. The Yellow Cardinal (Gubernatrix cristata) is a flagship species of this ecosystem, but it is classified as “critically endangered” in Brazil due to habitat loss and poaching for the illegal trade. Population supplementation through the release of individuals that were captive-bred or apprehended by authorities from the illegal trade has been considered as a conservation strategy for this species; however, the risk of pathogen introduction is a critical concern. We used microscopy and molecular methods to investigate the occurrence of blood parasites in wild passerines (n = 64, including three Yellow Cardinals) at Espinilho State Park, Rio Grande do Sul, Brazil, and in captive Yellow Cardinals (n = 30) at three facilities in Brazil. Haemosporidian parasites were detected in the blood smears of 10.9% of the wild passerines, comprising the morphospecies Haemoproteus erythrogravidus in Rufous-collared Sparrow (Zonotrichia capensis), H. quiscalus in Grayish Baywing (Agelaioides badius), and H. tyranni in Great Kiskadee (Pitangus sulphuratus); these are the southernmost records for these morphospecies and their first record for the Pampas biome. No haemosporidian parasites were detected in the blood smears of the Yellow Cardinals, wild or captive. Microfilariae were detected in the blood smears of 14.1% of the wild passerines, including all wild Yellow Cardinals, and in 43.3% of captive Yellow Cardinals. Trypanosoma sp. was detected in the blood smear of one captive Yellow Cardinal. Nested PCR and gene sequencing of the cyt-b gene of Haemoproteus/Plasmodium was used to test a subset of wild passerines and captive Yellow Cardinals, allowing for the molecular barcoding of H. quiscalus lineage AGEBAD04 and H. tyranni lineage PITSUL01; additionally, DNA identical to that of lineage PITSUL01 was detected in the blood of one captive Yellow Cardinal. This study provides valuable data to support the conservation management of the Yellow Cardinal and other threatened passerines from the Pampas and highlights the need for further studies on the epidemiology and pathology of filarioid worms and trypanosomes in passerines from this biome.





Similar content being viewed by others
Data availability
Genetic sequences produced in this study were deposited at GenBank (accession codes ON703102–4) and MalAvi (lineages AGEBAD04 and PITSUL01).
References
Atawal AF, Mgbeahuruike AC, Hammers M (2019) Microfilarial infections associated with body mass loss of Village Weavers Ploceus cucullatus. Ostrich 90:41–44. https://doi.org/10.2989/00306525.2018.1539418
Beier C, Repenning M, da Pereira MS et al (2017) Cooperative breeding and demography of Yellow Cardinal Gubernatrix cristata in Brazil. Rev Bras Ornitol 25:12–19. https://doi.org/10.1007/BF03544371
Bennett GF, Campbell A (1972) Avian Haemoproteidae. I. Description of Haemoproteus fallisi n. sp. and a review of the haemoproteids of the family Turdidae. Can J Zool 50:1269–1275
Bennett GF, Caines JR, Whiteway MA (1986) Avian Haemoproteidae. 20. The haemoproteids of the avian families Apodidae (swifts), Bucconidae (puffbirds), and Indicatoridae (honeyguides). Can J Zool 64:766–770. https://doi.org/10.1139/z86-113
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358
BirdLife International (2022) Species factsheet: Gubernatrix cristata. In: BirdLife International Data Zone. http://datazone.birdlife.org/species/factsheet/yellow-cardinal-gubernatrix-cristata. Accessed 6 Jun 2022
Burry-Caines JR, Bennett GF (1992) The Haemoproteidae (Apicomplexa: Haemosporina) of the avian families Fringillidae and Emberizidae s.l. Can J Zool 70:1149–1160. https://doi.org/10.1139/z92-161
Clark NJ, Adlard RD, Clegg SM (2015) Molecular and morphological characterization of Haemoproteus (Parahaemoproteus) ptilotis, a parasite infecting Australian honeyeaters (Meliphagidae), with remarks on prevalence and potential cryptic speciation. Parasitol Res 114:1921–1928
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772–772. https://doi.org/10.1038/nmeth.2109
de Castaneda RR, Morales J, Moreno Klemming J et al (2009) Costs and benefits of early reproduction: Haemoprotus prevalence and reproductive success of infected male pied flycatchers in montane habitat in Central Spain. Ardeola 56:271–280
de Oliveira L, Barino GTM, Rossi MF et al (2020) Morphological and molecular characterization of Haemoproteus coatneyi and Haemoproteus erythrogravidus (Haemosporida: Haemoproteidae) in Passeriformes in Brazil’s Atlantic Forest. Rev Bras Parasitol Veterinária 29:e011520. https://doi.org/10.1590/s1984-29612020074
De La Torre GM, Campião KM (2021) Bird habitat preferences drive hemoparasite infection in the Neotropical region. Integr Zool 16:755–768. https://doi.org/10.1111/1749-4877.12515
Dimitrov D, Valkiūnas G, Zehtindjiev P et al (2013) Molecular characterization of haemosporidian parasites (Haemosporida) in yellow wagtail (Motacilla flava), with description of in vitro ookinetes of Haemoproteus motacillae. Zootaxa 3666:369–381
Doussang D, González-Acuña D, Torres-Fuentes LG et al (2019) Spatial distribution, prevalence and diversity of haemosporidians in the rufous-collared sparrow Zonotrichia Capensis. Parasit Vectors 12:2. https://doi.org/10.1186/s13071-018-3243-4
Doussang D, Sallaberry-Pincheira N, Cabanne GS et al (2021) Specialist versus generalist parasites: the interactions between host diversity, environment and geographic barriers in avian malaria. Int J Parasitol 51:899–911. https://doi.org/10.1016/j.ijpara.2021.04.003
Durrant KL, Beadell JS, Ishtiaq F et al (2006) Avian hematozoa in South America: a comparison of temperate and tropical zones. Ornithol Monogr 60:98–111. https://doi.org/10.2307/40166831
Ellis VA, Fecchio A, Ricklefs RE (2020) Haemosporidian parasites of neotropical birds: causes and consequences of infection. Auk 137:ukaa055. https://doi.org/10.1093/auk/ukaa055
Fecchio A, Marini MÂ, Braga ÉM (2007) Baixa prevalência de hemoparasitos em aves silvestres no Cerrado do Brasil Central. Neotropical Biol Conserv 2:127–135
Fecchio A, Bell JA, Pinheiro RBP et al (2019) Avian host composition, local speciation and dispersal drive the regional assembly of avian malaria parasites in South American birds. Mol Ecol 28:2681–2693. https://doi.org/10.1111/mec.15094
González AD, Matta NE, Ellis VA et al (2014) Mixed species flock, nest height, and elevation partially explain avian haemoparasite prevalence in Colombia. PLoS ONE 9:e100695. https://doi.org/10.1371/journal.pone.0100695
Hamilton PB, Gibson WC, Stevens JR (2007) Patterns of co-evolution between trypanosomes and their hosts deduced from ribosomal RNA and protein-coding gene phylogenies. Mol Phylogenet Evol 44:15–25. https://doi.org/10.1016/j.ympev.2007.03.023
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802
Huang Y-L, Tsai S-S, Thongchan D et al (2017) Filarial nematode infection in eclectus parrots (Eclectus roratus) in Taiwan. Avian Pathol 46:188–194. https://doi.org/10.1080/03079457.2016.1237014
Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) (2018) Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Volume III – Aves. Ministério do Meio Ambiente, Brasília, Brazil. https://www.gov.br/icmbio/pt-br/centrais-de-conteudo/publicacoes/publicacoes-diversas/livro_vermelho_2018_vol3.pdf
Iezhova TA, Valkiūnas G, Loiseau C et al (2010) Haemoproteus cyanomitrae sp. nov. (Haemosporida: Haemoproteidae) from a widespread African songbird, the olive sunbird Cyanomitra Olivacea. J Parasitol 96:137–143
Jaramillo A (2020) Yellow Cardinal (Gubernatrix cristata). In: del Hoyo J, Elliott A, Sargatal J, de Juana E (eds) Birds of the world. Cornell Lab of Ornithology, Ithaca, NY. https://doi.org/10.2173/bow.yelcar1.01
Križanauskienė A, Pérez-Tris J, Palinauskas V et al (2010) Molecular phylogenetic and morphological analysis of haemosporidian parasites (Haemosporida) in a naturally infected European songbird, the blackcap Sylvia atricapilla, with description of Haemoproteus pallidulus sp. nov. Parasitology 137:217–227
Križanauskienė A, Iezhova TA, Palinauskas V et al (2012) Haemoproteus nucleocondensus n. sp. (Haemosporida, Haemoproteidae) from a Eurasian songbird, the Great Reed Warbler Acrocephalus arundinaceus. Zootaxa 3441:36–46
Kumar S, Stecher G, Li M et al (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Laird M, Van Riper C (1981) Questionable reports of Plasmodium from birds in Hawaii, with the recognition of P. relictum ssp. capistranoae (Russell, 1932) as the avian malaria parasite there. In: Canning EW (ed) Parasitological topics - a presentation volume to P.C.C. Garnham, F.R.S. on the occasion of his 80th birthday. Society of Protozoologists. Lawrence, Kansas, pp 159–165
Law JM, Tully TN, Stewart TB (1993) Verminous encephalitis apparently caused by the filarioid nematode Chandlerella quiscali in emus (Dromaius novaehollandiae). Avian Dis 37:597–601
Mantilla JS, González AD, Lotta IA et al (2016) Haemoproteus erythrogravidus n. sp. (Haemosporida, Haemoproteidae): description and molecular characterization of a widespread blood parasite of birds in South America. Acta Trop 159:83–94
Marchiori JNC, Alves FDS (2014) O inhanduvá (Prosopis affinis Spreng.) no Rio Grande do Sul. 8 – Aspectos fitogeográficos. Balduinia 0:13–20. https://doi.org/10.5902/2358198014133
Martinsen ES, Perkins SL, Schall JJ (2008) A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol 47:261–273
Martins-Ferreira C, Freitas TRO (2010) Genetic diversity and population structure of the endangered yellow cardinal Gubernatrix cristata and implications to its conservation efforts. In: Proceedings of the 25th International Ornithological Congress. International Ornithological Congress. Campos do Jordão, Brazil, p 868
Marzal A, Ricklefs RE, Valkiūnas G et al (2011) Diversity, loss, and gain of malaria parasites in a globally invasive bird. PLoS ONE 6:e21905
Nandi N, Bennett GF (1994) Re-description of Trypanosoma corvi Stephens and Christophers, 1908 emend. Baker, 1976 and remarks on the trypanosomes of the avian family Corvidae. Mem Inst Oswaldo Cruz 89:145–151
Olson DM, Dinerstein E, Wikramanayake ED et al (2001) Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51:933. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
Palinauskas V, Žiegytė R, Iezhova TA et al (2016) Description, molecular characterisation, diagnostics and life cycle of Plasmodium elongatum (lineage pERIRUB01), the virulent avian malaria parasite. Int J Parasitol 46:697–707. https://doi.org/10.1016/j.ijpara.2016.05.005
Paperna I, Yosef R (2010) Description of a new species of Haemoproteus (Haemosporidia, Apicomplexa) from an Orange-tufted Sunbird Nectarinia osea. Acta Parasitol 55:103–107
Piacentini VQ, Aleixo A, Agne CE et al (2015) Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee/Lista comentada das aves do Brasil pelo Comitê Brasileiro de Registros Ornitológicos. Rev Bras Ornitol 23:91–298
Redin CG, Longhi RV, Watzlawick LF, Longhi SJ (2011) Composição florística e estrutura da regeneração natural do Parque Estadual do Espinilho, RS. Ciênc Rural 41:1195–1201
Ribeiro SF, Sebaio F, Branquinho FCS et al (2005) Avian malaria in Brazilian passerine birds: parasitism detected by nested PCR using DNA from stained blood smears. Parasitology 130:261–267. https://doi.org/10.1017/S0031182004006596
Ronquist F, Teslenko M, Van Der Mark P et al (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Sebaio F, Braga ÉM, Branquinho F et al (2012) Blood parasites in passerine birds from the Brazilian Atlantic Forest. Rev Bras Parasitol Veterinária 21:7–15. https://doi.org/10.1590/S1984-29612012000100003
Serafini PP (2013) Plano de Ação Nacional para a Conservação dos Passeriformes Ameaçados dos Campos Sulinos e Espinilho. Instituto Chico Mendes de Conservação da Biodiversidade, Brasília, Brazil. https://www.icmbio.gov.br/cemave/images/stories/Publicações_científicas/livro-campos-sulinos-2013.pdf
Tostes R, Vashist U, Scopel KKG et al (2015) Plasmodium spp. and Haemoproteus spp. infection in birds of the Brazilian Atlantic Forest detected by microscopy and polymerase chain reaction. Pesqui Veterinária Bras 35:67–74. https://doi.org/10.1590/S0100-736X2015000100014
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton
Valkiūnas G, Z̆ic̆kus T, Shapoval AP, Lezhova TA (2006) Effect of Haemoproteus belopolskyi (Haemosporida: Haemoproteidae) on body mass of the blackcap Sylvia atricapilla. J Parasitol 92:1123–1125. https://doi.org/10.1645/GE-3564-RN.1
Valkiūnas G, Križanauskienė A, Iezhova TA et al (2007) Molecular phylogenetic analysis of circumnuclear hemoproteids (Haemosporida: Haemoproteidae) of sylviid birds, with a description of Haemoproteus parabelopolskyi sp. nov. J Parasitol 93:680–687. https://doi.org/10.1645/GE-1102R.1
Viggers K, Lindenmayer D, Spratt D (1993) The importance of disease in reintroduction programmes. Wildl Res 20:687. https://doi.org/10.1071/WR9930687
White EM, Greiner EC, Bennett GF, Herman CM (1978) Distribution of the hematozoa of Neotropical birds. Rev Biol Trop 26:43–102
Woodworth-Lynas CB, Caines JR, Bennett GF (1989) Prevalence of avian haematozoa in São Paulo state, Brazil. Mem Inst Oswaldo Cruz 84:515–526. https://doi.org/10.1590/S0074-02761989000400009
Zídková L, Cepicka I, Szabová J, Svobodová M (2012) Biodiversity of avian trypanosomes. Infect Genet Evol 12:102–112. https://doi.org/10.1016/j.meegid.2011.10.022
Acknowledgements
We wish to thank Secretaria Estadual do Meio Ambiente e Infraestrutura do Rio Grande do Sul (SEMA) and Espinilho State Park staff for permitting to research in a protected area and for all the support during fieldwork and Fazenda São Marcos owners for granting access to one of the study sites. We also thank Pai Passo farm Owners (especially Paulo, Pedro Bastos and Maria), Condomínio Agropecuário Ceolin, and Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for their huge support during field expeditions.
Funding
This study was supported by the ICMBio, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 422053/2016–3), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG APQ-00645–18). FCF was supported by the National Science Foundation grant 1717498 as part of the joint NSF-NIH-USDA Ecology and Evolution of Infectious Diseases program. CSF was supported by CNPq grant 309438/2016–0 and 310608/2019–8. EMB was granted by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 304334/2019–7).
Author information
Authors and Affiliations
Contributions
BRS, PPS, CSF, TWS, EC, and RLD conceived the project. BRS, PPS, CSF, TWS, and EC conducted field expeditions and collected and processed data and biological samples. BRS, RETV, FCF, and EMB examined blood smears and morphologically identified the parasites. AMC, FCF, RETV, and EMB conducted molecular and phylogenetic analyses. BRS, RETV, and RLD wrote the first manuscript draft and prepared the tables and figures. All authors reviewed and edited the manuscript and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the Animal Use Ethics Committee of the Agricultural Sciences Campus of the Universidade Federal do Paraná (CEUA 038/2018) and was conducted under permits issued by the Brazilian Ministry of Environment (SISBIO 53935–6).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Leonhard Schnittger.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva, B.R., Vanstreels, R.E.T., Serafini, P.P. et al. Blood parasites of passerines in the Brazilian Pampas and their implications for a potential population supplementation program for the endangered Yellow Cardinal (Gubernatrix cristata). Parasitol Res 121, 3203–3215 (2022). https://doi.org/10.1007/s00436-022-07638-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07638-w