Abstract
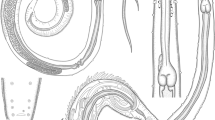
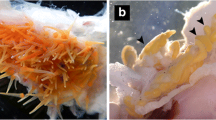
The present paper describes Filisoma argusum n. sp. (Cavisomatidae), an acanthocephalan parasite infecting the intestine of the spotted scat, Scatophagus argus (Linnaeus, 1766), in the south-west coast of India. The prevalence is 18% (mean intensity: 1.61 and abundance: 1–4 worms/host). Filisoma argusum n. sp. is morphologically characterized by a creamy-white, cylindrical, elongate, aspinose, and robust trunk; a collar-like neck; and a cylindrical proboscis with 18–20 longitudinal rows of hooks, with 19–22 hooks/row. Proboscis receptacle long, double-walled. Lemnisci digitiform, equal, longer than proboscis receptacle. Females 79.14 ± 33.69 × 0.593 ± 0.19 mm; males 32.62 ± 2.98 × 0.46 ± 0.071 mm. Males with four cement glands; bulbous muscular copulatory bursa with six digitiform rays. SEM studies revealed smooth hooks, sensory pits, and epidermal micropores. Histopathological changes at the site of parasite attachment included inflammation, hemorrhage, sloughing of epithelium, and detachment of mucosal layer of the intestine. In molecular and phylogenetic analyses, the parasite occupied an independent position within the Cavisomatidae clade with high bootstrap values for both ITS1-5.8S and ITS2, and mt-CO1 regions. Considering the morphologic and morphometric differences with previously described species of Filisoma along with its phylogenetic positioning, the present acanthocephalan is treated as a new species and the name Filisoma argusum n. sp. is proposed.







Similar content being viewed by others
References
Abd-El-Moaty SM, Taeleb AA (2011) Ultrastructural study on the body surface of Porrorchis indicus (Acanthocephala: Plagiorhynchidae) from the Egyptian cuculus, Centropus senegalensis aegyptius (Aves : Cuculidae). J Am Sci 7:571–577
Alcántar-Escalera FJ, García-Varela M, Vázquez-Domínguez E, Pérez-Ponce de León G (2013) Using DNA barcoding to link cystacanths and adults of the acanthocephalan Polymorphus brevis in central Mexico. Mol Ecol Resour 13(6):1116–1124. https://doi.org/10.1111/1755-0998.12090
Amin OM (2013) Classification of the Acanthocephala. Folia Parasitol 60:273–305
Amin OM, Heckmann RA (2012) An SEM study of Acanthogyrus (Acanthosentis) tilapiae (Acanthocephala: Quadrigyridae) from Africa documenting previously unreported features and host parasite interface. Sci Parasitol 13:57–63
Amin OM, Nahhas FM (1994) Acanthocephala of marine fishes off Fiji Islands, with descriptions of Filisoma longcementglandatus n. sp., Neorhadinorhynchus macrospinosus n. sp. (Cavisomatidae) and gravid females of Rhadinorhynchus johnstoni (Rhadinorhynchidae); and keys to species of the genera Filisoma and Neorhadinorhynchus. J Parasitol 80:768–774
Amin OM, Heckmann RA, Radwan NA, Anchundia JS, Alcivar MA (2009) Redescription of Rhadinorhynchus ornatus (Acanthocephala: Rhadinorhynchidae) from skipjack tuna, Katsuwonus pelamis, collected in the Pacific Ocean off South America, with special reference to new morphological features. J Parasitol 95:656–664
Amin OM, Heckmann RA, Ha NV (2014) Acanthocephalans from fishes and amphibians in Vietnam, with descriptions of five new species. Parasite 21(53):1–17
Bleeker P (1863) Description de quelques espèces de poissons, nouvelles ou peu connues de Chine, envoyées au Musée de Leide par M.-G. Schlegel. Nederlandsch Tijdschrift voor de Dierkunde 1:135–150
Bromham L, Cowman PF, Lanfear R (2013) Parasitic plants have increased rates of molecular evolution across all three genomes. BMC Evol Biol 13(126):1–11
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Datta MN, Soota TD (1962) Two new Acanthocephala from the fish Scatophagus argus (Bloch) from India. Rec Indian Museum 58(2):67–70
Dezfuli BS, Giari L, Simoni E, Bosi G, Manera M (2002) Histopathology, immune-histochemistry and ultrastructure of the intestine of Leuciscus cephalus (L.) naturally infected with Pomphorhynchus laevis (Acanthocephala). J Fish Dis 25:7–14
Dezfuli BS, Giovinazzo G, Lui A, Giari L (2008) Inflammatory response to Dentitruncus truttae (Acanthocephala) in the intestine of brown trout. Fish Shellfish Immunol 24:726–733
Edmonds SJ, Dixon BR (1966) Uptake of small particles by Moniliformos dubius (Acanthocephala). Nature 209:99
Emde S, Kochmann J, Kuhn T, Plath M, Klimpel S (2014) Getting what is served? Feeding ecology influencing parasite-host interactions in invasive round goby Neogobius melanostomus. PLoS One 9(10):e109971. https://doi.org/10.1371/journal.pone.0109971
Fernandes VSC, Amin OM, Borges JN, Santos CP (2019) A new species of the Acanthocephalan genus Filisoma (Cavisomatidae) from Perciform fishes in Rio de Janeiro, Brasil. Acta Parasitol 64(1):176–186. https://doi.org/10.2478/s11686-018-00019-3
Folmer O, Black M, Hoeh W, Lutz R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
García-Varela M, Pérez-Ponce de León G, Aznar FJ, Nadler SA (2009) Systematic position of Pseudocorynosoma and Andracantha (Acanthocephala, Polymorphidae) based on nuclear and mitochondrial gene sequences. J Parasitol 95:178–185
García-Varela M, Nadler SA (2005) Phylogenetic relationships of Palaeacantocephala (Acanthocephala) inferred from SSU and LSU rDNA gene sequences. J Parasitol 91:1401–1409. https://doi.org/10.1645/GE-523R.1
García-Varela M, Pérez-Ponce de León G (2008) Validating the systematic position of Profilicolus meyer, 1931 and Hexaglandula petrochenko, 1950 (Acanthocephala: Polymorphidae) using cytochrome c oxidase (cox1). J Parasitol 94:212–217
García-Varela M, Pérez-Ponce de Leon G, de la Torre P, Cummings MP, Sarma SS, Laclette JP (2000) Phylogenetic relationships of Acanthocephala based on analysis of 18S ribosomal RNA gene sequences. J Mol Evol 50:532–540. https://doi.org/10.1007/s002390010056
García-Varela M, Aznar FJ, de León GP, Pinero D, Laclette UP (2005) Molecular phylogeny of Corynosoma Lühe, 1904 (Acanthocephala), based on 5.8S and internal transcribed spacer sequences. J Parasitol 91:345–352
García-Varela M, Pérez-Ponce de Léon G, Aznar FJ, Nadler SA (2013) Phylogenetic relationship among genera of Polymorphidae (Acanthocephala), inferred from nuclear and mitochondrial gene sequences. Mol Phylogenet Evol 68:176–184
García-Varela M, Henández-Orts JS, Pinacho-Pinacho CD (2017) A morphological and molecular study of Pseudocorynosoma Aznar, Pérez Ponce de León and Raga 2006 (Acanthocephala: Polymorphidae) from Mexico with the description of a new species and the presence of cox 1 pseudogenes. Parasitol Int 66(2):27–36. https://doi.org/10.1016/j.parint.2016.11.007
George PV, Nadakal AM (1981) Observations on the intestinal pathology of the marine fish, Rachycentron canadus (Gunther) infected with the acanthocephalid worm Serrasentis nadakali (George & Nadakal, 1978). Hydrobiologia 78:59–62
Gibson D, Wayland M (2020) World List of marine Acanthocephala. Cavisomatidae Meyer, 1932. Accessed through:World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails & id= 20373 on 2020-11-22
Golvan YJ (1969) Systématique des Acanthocéphales (Acanthocephala Rudolphi, 1801). L’ordre des Palaeacanthocephala Meyer 1931. I. La super-famille des Echinorhynchoidea (Cobbold, 1876) Golvan et Houin 1963, Mémoire du Muséum National d’Histoire Naturelle, nouvelle série. A, Zoologie 57:1–373
Gupta S (2016) An overview on morphology, biology, and culture of spotted scat, Scatophagus argus (Linnaeus 1766). Rev Fish Sci Aquac 24(2):203–212. https://doi.org/10.1080/23308249.2015.1119800
Gupta NK, Jain M (1979) A note on Filisoma indicum Van Cleave, 1928. Indian J Parasitol 3:135–137
Gupta SP, Naqvi M (1986) On some acanthocephalan parasites of marine fishes from India and Pakistan. Indian J Helminthol 34(1):61–84
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hammond RA (1968) Observations of the body surface of some acanthocephalans. Nature 218:872–873
Harada I (1938) Acanthocephalen aus Formosa (1) Dr. A. OKA Jub. Annotationes Zoologicae Japonenses 17(3/4):419–427
Heckmann RA, Amin OM, AM EI-N (2013) Micropores of Acanthocephala, a scanning electron microscopy study. Sci Parasitol 14:105–113
Hernández-Orts JS, Smales LR, Pinacho-Pinacho CD, García-Varela M, Presswell B (2017) Novel morphological and molecular data for Corynosoma hannae Zdzitowiecki, 1984 (Acanthocephala: Polymorphidae) from teleosts, fish-eating birds and pinnipeds from New Zealand. Parasitol Int 66:905–916
Huston DC, Cribb TH, Smales LR (2020) Molecular characterisation of acanthocephalans from Australian marine teleosts: proposal of a new family, synonymy of another and transfer of taxa between orders. Syst Parasitol 97:1–23. https://doi.org/10.1007/s11230-019-09896-2
Irena VS, Damir V, Damir K, Zrinka D, Emil G, Helena C, Emin T (2013) Molecular characterisation and infection dynamics of Dentitruncus truttae from trout (Salmo trutta and Oncorhynchus mykiss) in Krka River, Croatia. Vet Parasitol 197:604–613
Kaur P, Shamal P, Chandran A, Binesh CP, Gishnu M, Asokan PK, Sanil NK (2017) Morphometric and molecular characterisation of Tenuiproboscis keralensis n. sp. infecting marine and brackish water fishes from the south-west coast of India with a note on morphological plasticity. Parasitol Res 116:3131–3149
Knudsen SW, Choat JH, Clements KD (2019) The herbivorous fish family Kyphosidae (Teleostei: Perciformes) represents a recent radiation from higher latitudes. J Biogeogr 00:1–14
Kral’ova-Hromadova I, Tietz DF, Shinn AP, Spakulova M (2003) ITS rDNA sequences of Pomphorhynchus laevis (Zoega in Muller, 1776) and P. lucyi Williams and Rogers, 1984 (Acanthocephala: Palaeacanthocephala). Syst Parasitol 56:141–145
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Lehnert K, Raga J, Siebert U (2007) Parasites in harbour seals (Phoca vitulina) from the German Wadden sea between two phocine distemper virus epidemics. Helgol Mar Res 61:239–245
Li L, Chen HX, Amin OM, Yang Y (2017a) Morphological variability and molecular characterization of Pomphorhynchus zhoushanensis sp. nov. (Acanthocephala: Pomphorhynchidae), with comments on the systematic status of Pomphorhynchus Monticelli, 1905. Parasitol Int 66(5):693–698. https://doi.org/10.1016/j.parint.2017.05.010
Li L, Yang Y, Zhang LP (2017b) Morphological and molecular study of Longicollum pagrosomi Yamaguti, 1935 (Acanthocephala: Pomphorhynchidae) from the barred knifejaw Oplegnathus fasciatus (Temminck & Schlegel) (Perciformes: Oplegnathidae) in the East China Sea. Syst Parasitol 94:255–261. https://doi.org/10.1007/s11230-016-9689-x
Martínez-Aquino A, Reyna-Fabián ME, Rosas-Valdez R, Razo-Mendivil U, de León GP, García-Varela M (2009) Detecting a complex of cryptic species within Neoechinorhynchus golvani (Acanthocephala: Neoechinorhynchidae) inferred from ITSs and LSU rDNA gene sequences. J Parasitol 95:1040–1047
Mills D, Vevers G (1989) The Tetra encyclopedia of freshwater tropical aquarium fishes. Tetra Press, New Jersey, 208 p
Nakao M (2016) Pseudoacanthocephalus toshimai sp. nov. (Palaeacanthocephala: Echinorhynchidae), a common acanthocephalan of anuran and urodelan amphibians in Hokkaido, Japan, with a fnding of its intermediate host. Parasitol Int 65:323–332. https://doi.org/10.1016/j.parint.2016.03.011
Nicholas WL, Mercer EH (1965) The infrastructure of the tegument of Moniliformis dubius (Acanthocephala). Q J Microsc Sci 106:137–146
Paterson S, Vogwill T, Buckling A, Benmayor R, Spiers AJ, Thomson NR, Quail M, Smith F, Walker D, Libberton B (2010) Antagonistic coevolution accelerates molecular evolution. Nature 11:275–278. https://doi.org/10.1038/nature08798
Patrick W, Kristina L, Ursula S, Iwona P, Christina S (2018) Prevalence and molecular characterisation of Acanthocephala in Pinnipedia of the North and Baltic Seas. Int J Parasitol Parasites Wildl 7(1):34–43
Pinacho-Pinacho CD, Hernández-Orts JS, Sereno-Uribe AL, Pérez-Ponce de León G, García-Varela M (2017) Mayarhynchus karlae n. g., n. sp. (Acanthocephala: Neoechinorhynchidae), a parasite of cichlids (Perciformes: Cichlidae) in southeastern Mexico, with comments on the paraphyly of Neoechinorhynchus Stiles & Hassall, 1905. Syst Parasitol 94:351–365
Presswell B, García-Varela M, Smales LR (2018) Morphological and molecular characterization of two new species of Andracantha (Acanthocephala: Polymorphidae) from New Zealand shags (Phalacrocoracidae) and penguins (Spheniscidae) with a key to the species. J Helminthol 92:740–751. https://doi.org/10.1017/S0022149X17001067
Rambaut A (2008) FigTree v. 1.4.0. Rambaut. Available from http://tree.bio.ed.ac.uk/software/figtree/.
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Sanil NK, Asokan PK, Lijo J, Vijayan KK (2011) Pathological manifestations of the acanthocephalan parasite, Tenuiproboscis sp. in the mangrove red snapper (Lutjanus argentimaculatus) (Forsskal, 1775), a candidate species for aquaculture from Southern India. Aquaculture 310:259–266
Schelhaas DP (1980) Comparative histopathology of Acanthocephan infections in some freshwater fishes. Thesis, University of North Dakota, M.Sc
Sepulveda MS, Kinsella JM (2013) Helminth collection and identification from wildlife. J Vis Exp (82):e51000. https://doi.org/10.3791/51000
Sheema SH, John MV, George PV (2017) SEM studies on acanthocephalan parasite, Echinorhynchus veli infecting the fish Synaptura orientalis (Bl & Sch, 1801). J Parasit Dis 41(1):71–75
Southwell T, Macfie S (1925) On a collection of Acanthocephala in the Liverpool School of Tropical Medicine. Ann Trop Med Par 19:141–184
Suvarna K, Layton C, Bancroft J (2018) Bancroft’s theory and practice of histological techniques - 8th Edition. Elsevier, United Kingdom, pp 126–139
Taraschewski H (2000) Host-parasite interactions in Acanthocephala: a morphological approach. Adv Parasitol 46:1–179
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tingbao Y, Xianghua L (2001) Seasonal population dynamics of Neoechinorhynchus qinghaiensis in the carp, Gymnocypris przewalskii przewalskii, from Qinghai Lake China. J Helminthol 75(1):93–98
Tkach VV, Lisitsyna OI, Crossley JL, Binh TT, Bush SE (2013) Morphological and molecular diferentiation of two new species of Pseudoacanthocephalus Petrochenko, 1958 (Acanthocephala: Echinorhynchidae) from amphibians and reptiles in the Philippines, with identification key for the genus. Syst Parasitol 85:11–26. https://doi.org/10.1007/s11230-013-9409-8
Tubangui MA, Masiluñgan VA (1946) On two acanthocephala from the Philippines. J Parasitol 32:144–155
Van Cleave HJ (1928) Two new genera and species of Acanthocephala from fishes of India. Rec Indian Museum 30:147–149
Van Cleave HJ (1940) Some comparisons of Acanthocephala of marine fishes of the Atlantic and Pacific coasts. J Parasitol 26(6):40–41
Van Cleave HJ, Manter HW (1947) A new species of the acanthocephalan genus Filisoma from the Dry Tortugas, Florida. J Parasitol 33:487–490
Wang YY, Wang PQ (1988) Notes on Acanthocephala from Fujian, with descriptions of three new species. J Fujian Normal Univ Natur Sci 3:80–86 (In Chinese)
Wang Y, Wang P, Wu D (1993) On some Echinorhynchoidea parasites from marine fishes of Fujian Province, China. Wuyi Sci J 10:29–39 (In Chinese)
Wanstall ST, Robotham PW, Thomas JS (1986) Pathological changes induced by Pomphorhynchus laevis Muller (Acanthocephala) in the gut of rainbow trout, Salmo gairdneri Richardson. Z Parasitenkd 72(1):105–114
Wayland MT, Vainio JK, Gibson DI, Herniou EA, Littlewood DT, Väinölä R (2015) The systematics of Echinorhynchus Zoega in Müller, 1776 (Acanthocephala, Echinorhynchidae) elucidated by nuclear and mitochondrial sequence data from eight European taxa. Zookeys 26(484):25–52. https://doi.org/10.3897/zookeys.484.9132
Weaver HJ, Smales LR (2013) Filisoma filiformis n. sp. (Echinorhynchida: Cavisomatidae), a new species of Acanthocephala from Kyphosus spp. (Perciformes: Kyphosidae) from the South Pacific, and a key to the genus Filisoma. Comp Parasitol 80(1):33–38
West AJ (1964) The acanthor membranes of two species of acanthocephalan. J Parasitol 50(6):731–734
Wongchinawit S, Sailasuta A, Paphavasit N (2007) Histological studies of the alimentary system in the adult spotted scat, Scatophagus argus Linnaeus, in mangrove forests of the Pak Phanang Estuary, Nakhon Si Thammarat Province. In: 11th BRT Annual Conference, October 15–18th Udonthani pp:241.
Yamaguti S (1954) Parasitic worms from Celebes. Part 8. Acanthocephala. Acta Med Okayama 8:406–413
Acknowledgements
The authors wish to thank the Director, Central Marine Fisheries Research Institute, Cochin and Indian Council of Agricultural Research, New Delhi, for providing necessary facilities for undertaking this work. The Authors also wish to thank Mr. K.M. David for the help rendered in preparing the line drawings.
Funding
The present study was supported by the Department of Science and Technology, New Delhi, through a Woman Scientist Scheme-A (WOSA) project for the first author (No.SR/ WOS-A/ LS-83/2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Federica Marcer
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, P., Shamal, P., Chandran, A. et al. Characterization of Filisoma argusum n. sp. (Acanthocephala: Cavisomatidae Meyer, 1932) infecting the spotted scat, Scatophagus argus (Linnaeus, 1766) from the Indian coast. Parasitol Res 120, 2505–2521 (2021). https://doi.org/10.1007/s00436-021-07207-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07207-7