Abstract
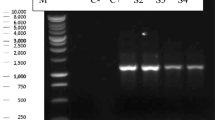
Recent phylogenetic and morphologic studies of Trichomonas spp. suggests that there are more than 3 species that infect the upper alimentary tract of wild birds, which include T. gallinae, T. stableri, and T. gypaetinii. In this study, investigations were conducted on the prevalence of trichomonads in the upper alimentary tract of 12 Steller’s sea eagles (Haliaeetus pelagicus) and 18 white-tailed sea eagles (H. albicilla). All birds were rescued from the wild and kept at a rehabilitation facility in Hokkaido, Japan, for variable durations and did not show any symptoms of trichomonosis. The ITS1-5.8SrRNA-ITS2 (ITS) genomic region of Trichomonas spp. was detected from 29 samples by PCR, and flagellates were confirmed from 4 samples by culture. Morphologic observations and measurement recordings were conducted under a light microscope on trophozoites obtained from the cultured isolates. Genomic sequences of the ITS, 18S ribosomal RNA (18S rRNA), Fe-hydrogenase, and RNA polymerase II largest subunit (Rpb1) regions were determined by direct sequencing, and phylogenetic analyses were conducted with previously published sequences of Trichomonas spp. All isolates were concluded as T. gypaetinii based on morphologic and molecular characterizations of the ITS and 18S rRNA genes. This is the first study to isolate T. gypaetinii from Haliaeetus eagles and further provide novel sequences of the Fe-hydrogenase and Rpb1 genes of T. gypaetinii. Both genomic regions also confirmed that T. gypaetinii belong to independent clusters from other Trichomonas spp.





Similar content being viewed by others
References
Amin A, Bilic I, Liebhart D, Hess M (2014) Trichomonads in birds - a review. Parasitology 141:733–747
Anderson NL, Grahn RA, Van Hoosear K, Bondurant RH (2009) Studies of trichomonad protozoa in free ranging songbirds: prevalence of Trichomonas gallinae in house finches (Carpodacus mexicanus) and corvids and a novel trichomonad in mockingbirds (Mimus polyglottos). Vet Parasitol 161:178–186
Boal CW, Mannan RW, Hudelson KS (1998) Trichomoniasis in Cooper’s hawks from Arizona. J Wildl Dis 34:590–593
Cepicka I, Kutisová K, Tachezy J, Kulda J, Flegr J (2005) Cryptic species within the Tetratrichomonas gallinarum species complex revealed by molecular polymorphism. Vet Parasitol 128:11–21
Chi JF, Lawson B, Durrant C, Beckmann K, John S, Alrefaei AF, Kirkbride K, Bell DJ, Cunningham AA, Tyler KM (2013) The finch epidemic strain of Trichomonas gallinae is predominant in British non-passerines. Parasitology 140:1234–1245
Dufernez F, Walker RL, Noël C, Caby S, Mantini C, Delgado-Viscogliosi P, Ohkuma M, Kudo T, Capron M, Pierce RJ, Villanueva MR, Viscogliosi E (2007) Morphological and molecular identification of non-Tritrichomonas foetus trichomonad protozoa from the bovine preputial cavity. J Eukaryot Microbiol 54(2):161–168
Felleisen RS (1997) Comparative sequence analysis of 5.8S rRNA genes and internal transcribed spacer (ITS) regions of trichomonadid protozoa. Parasitology 115:111–119
Forrester DJ, Foster GW (2008) Trichomonosis. In: Atkinson CT, Thomas NJ, Hunter DB (eds) Parasitic diseases of wild birds. Wiley-Blackwell, Ames, pp 120–153
Ganas P, Jaskulska B, Lawson B, Zadravec M, Hess M, Bilic I (2014) Multi-locus sequence typing confirms the clonality of Trichomonas gallinae isolates circulating in European finches. Parasitology 141:652–661
Gerhold RW, Yabsley MJ, Smith AJ, Ostergaard E, Mannan W, Cann JD, Fischer JR (2008) Molecular characterization of the Trichomonas gallinae morphologic complex in the United States. J Parasitol 94:1335–1341
Girard YA, Rogers KH, Gerhold R, Land KM, Lenaghan SC, Woods LW, Haberkern N, Hopper M, Cann JD, Johnson CK (2014a) Trichomonas stableri n. sp., an agent of trichomonosis in Pacific Coast band-tailed pigeons (Patagioenas fasciata monilis). Int J Parasitol Parasites Wildl 3:32–40
Girard YA, Rogers KH, Woods LW, Chouicha N, Miller WA, Johnson CK (2014b) Dual-pathogen etiology of avian trichomonosis in a declining band-tailed pigeon population. Infect Genet Evol 24:146–156
Grabensteiner E, Bilic I, Kolbe T, Hess M (2010) Molecular analysis of clonal trichomonad isolates indicate the existence of heterogenetic species present in different birds and within the same host. Vet Parasitol 172:53–64
Hess M, Kolbe T, Grabensteiner E, Prosl H (2006) Clonal cultures of Histomonas meleagridis, Tetratrichomonas gallinarum and a Blastocystis sp. established through micromanipulation. Parasitology 133:547–554
Inoue J, Saita K, Kudo T, Ui S, Ohkuma M (2007) Hydrogen production by termite gut protists: characterization of iron hydrogenases of Parabasalian symbionts of the termite Coptotermes formosanus. Eukaryot Cell 6:1925–1932
Kelly-Clark WK, McBurney S, Forzán MJ, Desmarchelier M, Greenwood SJ (2013) Detection and characterization of a Trichomonas isolate from a rehabilitated bald eagle (Haliaeetus leucocephalus). J Zoo Wildl Med 44:1123–1126
Lawson B, Cunningham A, Chantrey J, Hughes L, Kirkwood J, Pennycott T, Simpson V (2006) Epidemic finch mortality. Vet Rec 159:367
Lawson B, Cunningham AA, Chantrey J, Hughes LA, John SK, Bunbury N, Bell DJ, Tyler KM (2011) A clonal strain of Trichomonas gallinae is the aetiologic agent of an emerging avian epidemic disease. Infect Genet Evol 11:1638–1645
Lennon RJ, Dunn JC, Stockdale JE, Goodman SJ, Morris AJ, Hamer KC (2013) Trichomonad parasite infection in four species of Columbidae in the UK. Parasitology 140:1368–1376
Malik SB, Brochu CD, Bilic I, Yuan J, Hess M, Logsdon JM Jr, Carlton JM (2011) Phylogeny of parasitic Parabasalia and free-living relatives inferred from conventional markers vs. Rpb1, a single-copy gene. PLoS One 6:E20774
Martínez-Díaz RA, Ponce-Gordo F, Rodríguez-Arce I, del Martínez-Herrero MC, González FG, Molina-López RÁ, Gómez-Muñoz MT (2015) Trichomonas gypaetinii n. sp., a new trichomonad from the upper gastrointestinal tract of scavenging birds of prey. Parasitol Res 114:101–112
Martínez-Herrero MC, Sansano-Maestre J, López Márquez I, Obón E, Ponce C, González J, Garijo-Toledo MM, Gómez-Muñoz MT (2014) Genetic characterization of oropharyngeal trichomonad isolates from wild birds indicates that genotype is associated with host species, diet and presence of pathognomonic lesions. Avian Pathol 43:535–546
Martínez-Herrero MDC, González-González F, López-Márquez I, García-Peña FJ, Sansano-Maestre J, Martínez-Díaz RA, Ponce-Gordo F, Garijo-Toledo MM, Gómez-Muñoz MT (2019) Oropharyngeal trichomonosis due to Trichomonas gypaetinii in a cinereous vulture (Aegypius Monachus) fledgling in Spain. J Wildl Dis 55:153–157
Minowa K, Seki H, Suzuki N, Suzuki S, Terayama T, Mizoguchi I, Murakami M, Sugimori F, Iwama S, Nagahashi M, Ohi G (1982) Influence of lead on immunological response of pigeons (Columba livia var.) to the Trichomonas gallinae. Ann Rep Tokyo Metropol Res Lab Public Health 33:362–366
Oguma K (1931) On a species of Trichomonas parasitic on pigeon squabs. J Fac Sci Hokkaido Imp Univ 6:117–131
Pennycott T, Lawson B, Cunningham A, Simpson V, Chantrey J (2005) Necrotic ingluvitis in wild finches. Vet Rec 157:360
Robinson RA, Lawson B, Toms MP, Peck KM, Kirkwood JK, Chantrey J, Clatworthy IR, Evans AD, Hughes LA, Hutchinson OC, John SK, Pennycott TW, Perkins MW, Rowley PS, Simpson VR, Tyler KM, Cunningham AA (2010) Emerging infectious disease leads to rapid population declines of common British birds. PLoS One 5:e12215
Stabler RM (1954) Trichomonas gallinae: a review. Exp Parasitol 3:368–402
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729
Acknowledgements
Funding from the 2015 Akiyama Life Science Foundation is gratefully acknowledged. All samples were provided from the Ministry of the Environment, Government of Japan, Kushiro Nature Conservation Office. We would like to thank all the staff at the Institute for Raptor Biomedicine Japan Co. Ltd. for supporting the sampling of birds, which were humanely handled.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving animal were in accordance with the ethical standards of the institution at which the studies were conduct.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Berit Bangoura
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tomikawa, S., Nakagun, S., Watanabe, Y. et al. Molecular characterization of Trichomonas gypaetinii isolated from the upper alimentary tract of Steller’s sea eagles (Haliaeetus pelagicus) and white-tailed sea eagles (Haliaeetus albicilla) in Hokkaido, Japan. Parasitol Res 120, 2189–2198 (2021). https://doi.org/10.1007/s00436-021-07160-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07160-5