Abstract
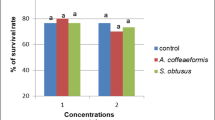
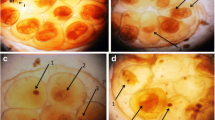
Schistosomiasis still affects a lot of people in many developing countries. Reducing the disease dissemination has been the target of various studies. As methyl gallate has antioxidant properties, it is assumed that it can be a good candidate for stimulating the immune response of snails. So, the aim of this work is to investigate the potential of using methyl gallate as an immunostimulant to Biomphalaria alexandrina snails in order to prevent the development of invading miracidia into infective cercariae. The infected snails were exposed to three concentrations of methyl gallate for two periods: 24 and 72 h. The results indicated that the most effective concentration was the lowest one: 125 mg/L of methyl gallate for 72 h, as it reduced both infection rate and mean number of shed cercariae. Also, it increased the total number of snails’ hemocytes in hemolymph, which were observed in head-foot region and digestive gland of treated snails surrounding degenerated sporocysts and cercariae. In addition, hydrogen peroxide showed its highest content in tissues of snails exposed to 125 mg/L of methyl gallate for 72 h. In conclusion, methyl gallate can be considered as one of the most promising immunostimulants of B. alexandrina snails against infection with Schistosoma mansoni.











Similar content being viewed by others
References
Abdel-Wareth MTA, Ghareeb MA (2018) Bioprospecting certain freshwater-derived fungi for phenolic compounds with special emphasis on antimicrobial and larvicidal activity of methyl gallate and p-coumaric acid. Egypt J Chem 61(5):773–784
Abou-El-Naga IF (2013) Biomphalaria alexandrina in Egypt: past, present and future. J Biosci 38:665–672. https://doi.org/10.1007/s12038-013-9329-4
Abou-El-Naga IF, Radwan EH (2012) Defense response of susceptible and resistant Biomphalaria alexandrina snails against Schistosoma mansoni infection. Revista de biología tropical 60(3):1195–1204
Adema CM, van Deutekom-Mulder EC, Van Der Knaap WPW, Sminia T (1994) Schistosomicidal activities of Lymnaea stagnalis haemocytes: the role of oxygen radicals. Parasitol 109(4):479–485
Anderson RM, Mercer JG, Wilson RA, Carter NP (1982) Transmission of Schistosoma mansoni from man to snail: experimental studies of miracidial survival and infectivity in relation to larval age, water temperature, host size and host age. Parasitol 85:339–360
Bahgat DM, Mossalem HS, Al-Sayed E, Eldahshan OA, Singab AN, Abu El Einin H (2018) Influence of saponin fraction from Albizia anthelmintica on Biomphalaria alexandrina snail; the intermediate host of Schistosoma mansoni in Egypt. Egypt J Aqua Biol Fish 22(5):231–240
Bakry FA (2009) Effect of infection with Schistosoma mansoni on some biological parameters in Biomphalaria alexandrina snails. Amer Eur J Sci Res 4(3):180–190
Barçante TA, Barçante JMP, Fujiwara RT, Lima WS (2012) Analysis ofcirculating haemocytes from Biomphalaria glabrata following Angiostrongylus vasorum infection using flow cytometry. J Parasitol Res https://doi.org/10.1155/2012/314723
Barracco MA, Steil AA, Gargioni R (1993) Morphological characterization of the hemocytes of the pulmonate snail Biomphalaria tenagophila. Memórias do Instituto Oswaldo Cruz 88(1):73–83
Bayne CJ, Buckley PM, DeWan PC (1980) Macrophagelike hemocytes of resistant Biomphalaria glabrataare cytotoxic for sporocysts of Schistosoma mansoni in vitro. J Parasitol 66:413–419
Bender RC, Broderick EJ, Goodall CP, Bayne CJ (2005) Respiratory burst of Biomphalaria glabrata hemocytes: Schistosoma mansoni–resistant snails produce more extracellular H2O2 than susceptible snails. J Parasitol 91(2):275–279
Bezerra FSM, Nogueira-Machado JA, Chaves MM, Correa RF, Coelho PMZ (2003) Effect of gamma radiation on the activity of hemocytes and the course of Schistosoma mansoni in resistant Biomphalaria glabrata. Mem. Inst Oswaldo Cruz 98:73–75
Borges CM, Souza CP, Andrade ZA (1998) Histopathological features associated with susceptibility and resistance of Biomphalaria snails to infection with Schistosoma mansoni. Mem Inst Oswaldo Cruz 93:117–121
Chaudhuri D, Ghate NB, Singh SS, Mandal N (2015) Methyl gallate isolated from Spondias pinnata exhibits anticancer activity against human glioblastoma by induction of apoptosis and sustained extracellular signal-regulated kinase 1/2 activation. Pharmaco Magaz 11:269–277
Coustau C, Gourbal B, Duval D, Yoshino TP, Adema CM, Mitta G (2015) Advances in gastropod immunity from the study of the interaction between the snail Biomphalaria glabrata and its parasites: a review of research progress over the last decade. Fish Shellfish Immunol 46(1):5–16
dos Santos AF, Fonseca SA, César FA, Albuquerque MC, Santana JV, Santana AE (2014) A penta-substituted pyridine alkaloid from the rhizome of Jatropha elliptica (Pohl) Muell. Arg. is active against Schistosoma mansoni and Biomphalaria glabrata. Parasitol Res 013:37–43
Ekaprasada MT, Nurdin H, Ibrahim S, Dachriyanus D (2010) Antioxidant activity of methyl gallate isolated from the leaves of Toona sureni. Indonesian J Chem 9:457–460
Ekaprasada MT, Nurdin H, Ibrahim S, Dachriyanus D (2015) Antibacterial activity of methyl gallate isolated from the leaves of Toona sureni. Int J Adv Sci Engineer Info Technol 5:280–282
El Sayed KA, Mahmoud MB, Mossalem HS (2011) Cryptostegia grandiflora affecting compatibility of Biomphalaria alexandrina and Biomphalaria glabrata to infection with Schistosoma mansoni with emphasis on some hematological effects. Aust J Basic Appl Sci 5(12):3357–3365
El Sayed K, Soliman MG, Elfekky FA, Ouf NAA, Gad RM (2017) Susceptibility of Biomphalaria alexandrina snails to infection with Schistosoma mansoni miracidia under the effect of sodium alginates as immunostimulant. Europ J Biom 4(05):115–123
El-Sheikha HM, Hussein HS, Rahbar MH (2008) Clinico-pathological effects of Schistosoma mansoni infection associated with simultaneous exposure to malathion in Swiss outbred albino mice. Acta Trop 108:11–19
Feng SY, Feng JS, Burke CN, Khairallah LH (1971) Light and electron microscopy of the leucocytes of Crassostrea virginica (Mollusca: Pelecypoda). Z Zellforsch 120:222–245
Fossati P, Prencipe L, Berti G (1983) Enzymic creatinine assay: a new colorimetric method based on hydrogen peroxide measurement. Clin Chem 29(8):1494–1496
Haggag AA, Rabiee A, Elaziz KMA, Gabrielli AF, Hay RA, Ramzy RM (2017) Mapping of Schistosoma mansoni in the Nile Delta, Egypt: assessment of the prevalence by the circulating cathodic antigen urine assay. Acta Trop 167: 9–17. 10.1016/j. actatropica.2016.11.038
Hahn UK, Bender RC, Bayne CJ (2000) Production of reactive oxygen species by hemocytes of Biomphalaria glabrata: carbohydrate-specific stimulation. Developm Comp Immunol 24(6-7):531–541
Hahn UK, Bender RC, Bayne CJ (2001a) Killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata: role of reactive oxygen species. J Parasitol 87(2):292–299
Hahn UK, Bender RC, Bayne CJ (2001b) Involvement of nitric oxide in killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata. J Parasitol 87(4):778–785
Helal I, EL Mehlawy M, Rizk E, El-Khodary G (2003) Effect of Euphorbia peplus plant extract and the antihelmenthic prazequantel on the defence system of Biomphalaria alexandria snail. Egypt J Aqua Biol Fish 7(4):501–527
Helal EG, El-Dafrawy SM, Mohamed AH, Abou-El-Nour BM, Ibrahim S (2014) Ultrastructural study on Biomphalaria alexandrina hemocytes infected with Schistosoma mansoni in Egypt and its correlation with nitric oxide level. J Egypt Soc Parasitol 44:113–124
Humphries JE, Yoshino TP (2008) Regulation of hydrogen peroxide release in circulatinghemocytes of the planorbid snail Biomphalaria glabrata. Develop Comp Immunol 32:554–562. https://doi.org/10.1016/j.dci.2007.09.001
Hussein RM, Marie MAS, El-Deeb FAA, Hasheesh W, Sayed SSM (2016) Effects of three inorganic fertilizers on the biology and histopathology of infected Biomphalaria alexandrina snails. Res J Pharm Biol Chem Sci 7:2564–2574
Ibrahim AM, Abdel-Tawab H (2020) Cystoseira barbata marine algae have a molluscicidal activity against Biomphalaria alexandrina snails supported by scanning electron microscopy, hematological and histopathological alterations, and larvicidal activity against the infective stages of Schistosoma mansoni. Biologia 1-10.
Kamatham S, Kumar N, Gudipalli P (2015) Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol Rep 2:520–529
King CH, Sturrock RF, Kariuki HC, Hamburger J (2006) Transmission control for schistosomiasis-why it matters now. Trends Parasitol 22:575–582
Kiros G, Erko B, Giday M, Mekonnen Y (2014) Laboratory assessment of molluscicidal and cercariacidal effects of Glinus lotoides fruits. BMC Res Notes 7:220–227
Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N (2006) Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 20:593–600
Little J, Hultmark D, Read AF (2005) Invertebrate immunity and the limits of mechanistic immunology. Nat. Immunol. 6:651–654
Loker ES, Bayne CJ, Buckley PM, Kruse KT (1982) Ultrastructure of encapsulation of Schistosoma mansoni mother sporocysts by hemocytes of juveniles of the 10-R2 strain of Biomphalaria glabrata. J parasitol 68:84–94
Loker ES, Adema CM, Zhang SM, Kepler TB (2004) Invertebrate immune systems – not homogeneous, not simple, not well understood. Immunol Rev 198:10–24
Lotfy WM, DeJong RJ, Abdel Kader AA, Loker ES (2005) A molecular survey of Biomphalaria in Egypt: is B. glabrata present? Amer. J. Trop. Med. Hyg. 73:131–139
Martins-Souza RL, Pereira CA, Martins Filho OA, Coelho PMZ, Correa A (2006) Differential lectin labeling of circulating hemocyte from Biomphalaria glabrata and Biomphalaria tenagophila resistant or susceptible to Schistosoma mansoni infection. Mem Inst Oswaldo Cruz 101:185–192
Mohamed AH, Sharaf El-Din AT, Mohamed AM, Habib MR (2012) The relationship between genetic variability and the susceptibility of Biomphalaria alexandrina snails to Schistosoma mansoni infection. Mem. Inst. Oswaldo Cruz 107: 326–337. 10.1590/S0074-02762012000300006
Molla E, Giday M, Erko B (2013) Laboratory assessment of the molluscicidal and cercariacidal activities of Balanites aegyptiaca. Asian Pac J Trop Biomed 3:657–662
Mossalem HS, Mossa ATH (2014) Effect of rice bran extract on immunological and physiological parameters of Biomphalaria alexandrina snails infected with Schistosoma mansoni. Afr J Pharm Pharmacol 8(22):621–628
Mossalem HS, Ghareeb MA, Refahy LA, Mohamed AS, Habib MR (2017) Gas chromatography-mass spectrometry analysis and antioxidant activity of Punica granatum L. peels and its role as immunostimulant against Schistosoma mansoni infection in Biomphalaria alexandrina. GAS, 10 (1)
Mossalem HS, Habib MR, Ghareeb MA (2018) Control of infection of Biomphalaria alexandrina (Ehrenberg, 1831) with Schistosoma mansoni Sambon, 1907 using Eucalyptus camaldulensis. Folia Malacologica 26(3):155–165
Negrão-Corrêa D, Pereira CAJ, Rosa FM, Martins-Souza RL, Andrade ZA, Coelho PMZ (2007) Molluscan response to parasite, Biomphalaria and Schistosoma mansoni interaction. Inverteb Surv J 4:101–111
Nelwan M. L. (2019) Schistosomiasis: life cycle, diagnosis, and control. Curr Ther Res Clin Exp 91: 5–9. 10.1016/j.curtheres.2019.06.001
Obare B, Yole D, Nonoh J, Lwande W (2016) Evaluation of cercaricidal and miracicidal activity of selected plant extracts against larval stages of Schistosoma mansoni. JNSR 6(22):24–31
Osman GY, Mohamed AZ, Sheir KS, Hassab EL, Nabi SE, Allam AS (2014) Molluscidal activity of Mirazid on Biomphalaria alexandrina snails: biological and molecular studies. Inter J Adv Res 2(2):977–989
Rang HP, Dale MM, Flower RJ, Henderson G (2015) Rang and Dale's pharmacology (Eighth ed.). United Kingdom: Elsevier Churchill Livingstone.
Rollinson D, Webster JP, Webster B, Nyakaana S, Jorgensen A (2009) Genetic diversity of schistosomes and snails: implications for control. Parasitol:1–11
Saad AA, Khalil MT, Ragab FM, Mekawey AA, Abdel-Wareth MTA (2016) Separation of a compound effective against Biomphalaria alexandrina snails from the filtrate of Penicillium janthinellum. Inter J Environ Stud 73:1–17
Saad AH, El Einin HA, Abdel-Gaber R, Ayoub M, Mansour S (2017) Assessment of Bulinus truncatus immune response against Schistosoma haematobium infection by tissue reaction and hemocytes count. Res J Pharm Biol Chem Sci 8(3):1186–1196
Soliman MG, El Sayed K, Abou Ouf NA, El Fekky FAEH, Gad RM (2017) Influence of immunostimulatory β-glucan on Biomphalaria alexandrina snails under laboratory and simulated field conditions. Europ J Biom 4(12):41–50
Souza SD, Andrade ZA (2012) The significance of the amoebocyte-producing organ in Biomphalaria glabrata. Mem Inst Oswaldo Cruz 107(5):598–603
Souza CPD, Borges CC, Santana AG, Andrade ZA (1997) Comparative histopathology of Biomphalaria glabrata, B. tenagophila and B. straminea with variable degrees of resistance to Schistosoma mansoni miracidia. Memórias do Instituto Oswaldo Cruz 92: 517-522.
Sparks AK (1972) Invertebrate pathology: noncommunicable diseases. (1st eds), Academic Press, New York, USA
Sritunyalucksana K, Sithisarn P, Withayachumnarnkul B, Flegel TW (1999) Activation of prophenoloxidase, agglutinin and antibacterial activity in haemolymph of the black tiger prawn, Penaeus monodon, by immunostimulants. Fish Shellfish Immunol 9(1):21–30
Van der Knaap WPW, Loker ES (1990) Immune mechanisms in trematode-snail interactions. Parasitol Today 6(6):175–182
WHO (2013) World Health Organization. Schistosomiasis: progress report 2001–2011 and strategic plan 2012–2020. Geneva, Switzerland
WHO (2016) World Health Organization. Schistosomiasis: number of people treated worldwide in 2014. Weekly Epidemiological Record 91: 53–60. http://www.who.int/wer/2016/wer9105.
Wu GY, Halim MH (2000) Schistosomiasis: progress and problems. World J Gastroenterol 6(1):12–19. https://doi.org/10.3748/wjg.v6.i1.12
Yoshino TP, Coustau C (2011) Immunobiology of Biomphalaria-trematode interactions. In: Biomphalaria snails and larval trematodes. New York: Springer 159-89.
Yousif F, Ibrahim A, El-Bardicy SN (1998) Compatibility of Biomphalaria alexandrina, Biomphalaria glabrata and a hybrid of both to seven strains of Schistosoma mansoni from Egypt. J Egypt Soc Parasitol 28:863–881
Zelck U, Becker W (1992) Biomphalaria glabrata: influence of calcium, lectins, and plasma factors on in vitro phagocytic behavior of hemocytes of noninfected or Schistosoma mansoni-infected snails. Exper parasitol 75(1):126–136
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national, and international guidelines for the care and use of animals were followed.
Additional information
Section Editor: Christoph G. Grevelding
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mansour, S.M., Sayed, S.S.M. & Abdel-Wareth, M.T.A. Effect of methyl gallate on immune response of Biomphalaria alexandrina (Ehrenberg, 1831) snails to infection with Schistosoma mansoni (Sambon, 1907). Parasitol Res 120, 1011–1023 (2021). https://doi.org/10.1007/s00436-020-07037-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-07037-z