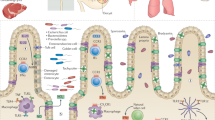
Abstract
Parasitic diseases affect more than one billion people worldwide, and most of them are chronic conditions in which the treatment and prevention are difficult. The appearance of granulomas, defined as organized and compact structures of macrophages and other immune cells, during various parasitic diseases is frequent, since these structures will only form when individual immune cells do not control the invading agent. Th2-typering various parasitic diseases are frequent, since these structures will only form when individual immune cells do not control the invading agent. The characterization of granulomas in different parasitic diseases, as well as recent findings in this field, is discussed in this review, in order to understand the significance of the granuloma and its modulation in the host–parasite interaction and in the immune, pathological, and parasitological aspects of this interaction. The parasitic granulomatous diseases granulomatous amebic encephalitis, toxoplasmosis, leishmaniasis, neurocysticercosis, and schistosomiasis mansoni are discussed as well as the mechanistic and dynamical aspects of the infectious granulomas.

Similar content being viewed by others
References
Abath FGC, Morais CNL, Montenegro CE, Wynn TA, Montenegro SM (2006) Immunopathogenic mechanisms in schistosomiasis: what can be learnt from human studies? Trends Parasitol 22:85–91
Abdulla MC, Jemshad A (2017) Bone marrow granuloma due to toxoplasmosis presenting as isolated thrombocytopenia in na immunocompetent patient. Egypt J Haematol 42:78–79
Abdulla MH, Lim KC, McKerrow JH, James H, Caffrey CR (2011) Proteomic identification of IPSE/alpha-1 as a major hepatotoxin secreted by Schistosoma mansoni eggs. PLoS Negl Trop Dis 5:e1368–e1377
Adalid-Peralta L, Arce-Sillas A, Fragoso G, Cárdenas G, Rosetti M, Casanova-Hernández D, Rangel-Escareno C, Uribe-Figueroa L, Fleury A, Sciutto E (2013) Cysticerci drive dendritic cells to promote in vitro and in vivo Tregs differentiation. Clin Dev Immunol 2013:981468
Adams DO (1976) The granulomatous inflammatory response. Am J Pathol 84:164–192
Almeida RP, Barral-Netto M, Jesus AMR, Freitas LAR, Carvalho EM, Barral A (1996) Biological behavior of Leishmania amazonensis isolated from humans with cutaneous, mucosal or visceral leishmaniasis in Balb/c mice. Am J Trop Med Htg 54:178–184
Alvarez JI, Londoño DP, Alvarez AL, Trujillo J, Jamarillo MM, Restrepo BI (2002) Granuloma formation and parasite disintegration in porcine cysticercosis: comparison with human neurocysticercosis. J Comp Pathol 127:186–193
Amaral KB, Silva TP, Dias FF, Malta KK, Rosa FM, Costa-Neto SF, Gentile R, Melo RCN (2017) Histological assessment of granulomas in natural and experimental Schistosoma mansoni infections using whole slide imaging. PLoS One 12:e0184696–e0184715
Anderson CF, Mendez S, Sacks DL (2005) Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J Immunol 174:2934–2941
Andrade ZA (1987) Pathology of human schistosomiasis. Mem Inst Oswaldo Cruz 82:17–23
Andrade ZA (2009) Schistosomiasis and liver fibrosis. Parasite Immunol 31:656–663
Aoun J, Habib R, Charaffeddine K, Taraif S, Loya A, Khalifeh I (2014) Caseating granulomas in cutaneous leishmaniasis. PLoS Negl Trop Dis 8:e325
Aráujo AP, Frezza TF, Alegretti SM, Giorgio S (2010) Hypoxia, hypoxia-inducible factor 1a and vascular endothelial growth factor in a murine model of Schistosoma mansoni infection. Exp Mol Pathol 89:327–333
Arechavaleta F, Molinari JL, Tato P (1998) A Taenia solium metacestode factor nonspecifically inhibits cytokine production. Parasitol Res 84:117–122
Arruda MSP, Nogueira MES, Bordon AP (2002) Histological evaluation of the lesion induced by inoculation of Leishmania mexicana in the cheek pouch of the hamster. Ver Soc Bras Med Trop 35:293–297
Baig AM (2015) Pathogenesis of amoebic encephalitis: are the amoebae being credited to an ‘inside job’ done by the host immune response? Acta Trop 148:72–76
Beattie L, Peltan A, Maroof A (2010) Dynamic imaging of experimental Leishmania donovani-induced hepatic granulomas detects Kupffer cell-restricted antigen presentation to antigen-specific CD8+ T Cells. PLoS Pathog 6:e1000805
Belfort RN, Rasmussen S, Kherani A, Lodha N, Williams J, Fernandes BF, Burnier MN Jr (2010) Bilateral progressive necrotizing retinochoroiditis in an immunocompromised patient: histopathological diagnosis. Acta Ophthalmol 88:614–615
Bertoli F, Espino M, Arosemena JR, Fishback JL, Frenkel JK (1995) A spectrum in the pathology of toxoplasmosis in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med 119:214–224
Betson M, Sousa-Figueiredo JC, Rowell C, Kabatereine N, Stothard JR (2010) Intestinal schistosomiasis in mothers and young children in Uganda: investigation of field-applicable markers of bowel morbidity. Am J Trop Med Hyg 83:1048–1055
Bogdan C, Donhauser N, Döring R, Rollinghoff M, Diefenbach A, Ritig MG (2000) Fibroblasts as host cells in latent leishmaniosis. J Exp Med 191:2121–2130
Boros LD (1986) Experimental granulomatosis. Clin Dermatol 4:10–12
Burke ML, Jones MK, Gobert GN (2009) Immunopathogenesis of human schistosomiasis. Parasite Immunol 31:163–176
Burza S, Croft SL, Boelaert M (2019) Leishmaniasis. Lancet 393:951–970
Cáceres-Dittmar G, Tapia FJ, Sanchez MA, Yamamura M, Uyemura K, Modlin RL, Bloom BR, Convit J (1993) Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin Exp Immunol 91:500–505
Cao J, Liu WJ, Xu XY, Zou XP (2010) Endoscopic findings and clinicopathologic characteristics of colonic schistosomiasis: a report of 46 cases. World J Gastroenterol 16:723–727
Carpio A, Fleury A, Romo ML, Abraham R (2018) Neurocysticercosis: the good, the bad, and the missing. Expert Ver Neurother 18:289–301
Chen L, Christian DA, Kochanowsky JA, Phan AT, Clark JT, Wang S, Berry C, Oh J, Chen X, Roos DS, Beiting DP, Koshy AA, Hunter CA (2020) The Toxoplasma gondii virulence factor ROP16 acts in cis and trans, and suppresses T cell responses. J Exp Med 217:e20181757
Chensue SW (2013) Chemokines in innate and adaptative granuloma formation. Front Immunol 4:43. Chuah C, Jones MK, Burke ML, McManus DP, Gobert GN (2014) Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol 30:141–150
Chuah C, Jones MK, Burke ML, McManus DP, Gobert GN (2014) Cellular and chemokine-mediated regulation in schistosome-induce hepatic pathology. Trends Parasitol 30:141–150
Colley DG, Bustinduy AL, Secor WE, King C (2014) Human schistosomiasis. Lancet 383:2253–2264
Commodaro AG, Belfort RN, Rizzo LV, Muccioli C, Silveira C, Burnier MM Jr, Belfort R Jr (2009) Ocular toxoplasmosis–an update and review of the literature. Mem Inst Oswaldo Cruz 104:345–350
Corrêa G, Lindenberg CA, Moreira-Souza ACA, Savio LEB, Takiya CM, Marques-da-Silva C, Vommaro RC, Coutinho-Silva R (2017) Inflammatory early events associated to the role of P2X7 receptor in acute murine. Immunobiology 222:676–683
Corte-Real S, Santos CB, Meirelles MN (1995) Differential expression of the plasma membrane Mg2+ ATPase and Ca2+ ATPase activity during adhesion and interiorization of Leishmania amazonensis in fibroblasts in vitro. J Submicrosc Cytol Pathol 27:359–366
Costain AH, MacDonald AS, Smits HH (2018) Schistosome egg migration: mechanisms, pathogenesis and host immune responses. Front Immunol 9:3042–3057
Cotterell SEJ, Engwerda CR, Kaye PM (1999) Leishmania donovani infection initiates T cell-independent chemokine responses, which are subsequently amplified in a T cell-dependent manner. Eur J Immunol 29:203–214
De Cook KM (1986) Hepatosplenic schistosomiasis: a clinical review. Gut 27:734–745
De Jesus AR, Silva A, Santana LB, Magalhães A, Jesus AM, Almeida RP, Rêgo MAV, Burattini MN, Pearce EJ, Carvalho EM (2002) Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J Infect Dis 185:98–105
De Lima DCV, Santos AS, Da Silva LTR, Melo RPB, Silva AG, Pinheiro JW Jr, Mota RA (2016) Occurrence of Toxoplasma gondii in domestic rabbits of Northeastern Brazil. Acta Parasitol 61:500–507
Del Brutto OH (2014) Neurocysticercosis. Neurohospitalist 4:205–212
Denkers EY, Gazzinelli RT (1998) Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Ver 4:569–588
Díaz A, Sagasti C, Casaravilla C (2017) Granulomatous responses in larval taeniid infectious. Parasite Immunol 40(5):e12523
Dubey JP (2008) The history of Toxoplasma gondii-the first 100 years. J Eukaryot Microbiol 55:467–475
Duggal SD, Rongpharpi SR, Duggal AS, Kumas A, Biswal I (2017) Role of Acanthamoeba in granulomatous encephalitis: a review. J Infect Dis Immune Ther 1:1. https://doi.org/10.4172/JIDITH.1000103 Corpus ID: 56064175
El-shoura SM, Tallab T, Bahamdan K (1996) Human cutaneous leishmaniasis: ultrastructural interactions between the inflammatory cells and Leishman bodies in the skin lesions. Parasite J 3:229–236
Escobar A (1983) The pathology of neurocysticercosis. In: Palacios E, Rodriguez-Carbajal, Taveras J, editors. Cysticercosis of the central nervous system. Springfield 27-54.
Falangola MF, Reichler BS, Petito CK (1994) Histopathology of cerebral toxoplasmosis in human immunodeficiency virus infection: a comparison between patients with early-onset and late-onset acquired immunodeficiency syndrome. Hum Pathol 25:1091–1097
Falzone C, Baroni M, De Lorenzi D, Mandara MT (2008) Toxoplasma gondii brain granuloma in a cat: diagnosis using cytology from na intraoperative sample and sequential magnetic resonance imaging. J Small Anim Pract 49:95–99
Filipe-Santos O, Pescher P, Breart B, Lippuner C, Aebischer T, Glaichenhaus N, Späth GF, Bousso P (2009) A dynamic map of antigen recognition by CD4 T Cells at the site of Leishmania major infection. Cell Host Microbe 6:23–33
Fleury A, Cardenas G, Adalid-Peralta L, Fragoso G, Sciutto E (2016) Immunopathology in Taenia solium neurocysticercosis. Parasite Immunol 38:147–157
García HH, Gonzalez A, Evans CAW, Gilman RH (2003) Taenia solium cysticercosis. Lancet 362:547–556
García HH, Gonzalez AE, Rodriguez S, Tsang VCW, Pretell EJ, Gozales I, Gilman RH, Cysticercosis Working Group in Peru (2010) Neurocysticercosis. Unraveling the nature of the single cysticercal granuloma. Neurology 75:654–658
García HH, O’Neal SE, Noh J, Handali S, Cysticercosis Working Group in Peru (2018) Laboratory diagnosis of neurocysticercosis (Taenia solium). J Clin Microbiol 56:e00424–e00418
Ghoname SI, Mohamed AH, El-Dafrawy SM (2017) Immune response of stimulated Biomphalaria alexandrina snails with Schistosoma mansoni infection. J Egypt Soc Parasitol 47:219–225
Giorgio S, Linares E, Ischiropoulos H, Von Zuben FJ, Yamada A, Augusto O (1998) In vivo formation of electron paramagnetic resonance-detectable nitric oxide and nitrotyrosine is not impaired during murine leishmaniasis. Infect Immun 66:807–814
Goto H, Prianti MG (2009) Immunoactivation and immunopathogeny during active visceral leishmaniasis. Rev Inst Med Trop S Paulo 5:241–246
Guarner J (2012) Detection of microorganisms in granulomas that have been formalin-fixed: review of the literature regarding use of molecular methods. Scientifica 2012: -16
Guarner J, Bartlett J, Shieh W-J, Paddock CD, Visvesvara GS, Zaki SR (2007) Histopathological spectrum and immunohistochemical diagnosis of amebic meningoencephalitis. Mod Pathol 20:1230–1237
Guiraldo E, Schlesinger LS (2013) Modeling the Mycobacterium tuberculosis granuloma–the critical battlefield in host immunity and disease. Front Immunol 4:98. https://doi.org/10.3389/fimmu.2013.00098
Hagen J, Young ND, Every AL, Pagel CN, Schnoeller C, Scheerlinck JP, Gasser RB, Kalinna BH (2014) Omega-1 knockdown in Schistosoma mansoni eggs by lentivirus transduction reduces granuloma size in vivo. Nat Commun 5:5375
Hams E, Aviello G, Fallon PG (2013) The Schistosoma granuloma: friend or foe? Front Immunol 4:1–7
Harrison JL, Ferreira GA, Raborn ES, Lafrenaye D, Marciano-Cabral F, Cabral GA (2010) Acanthamoeba culbertsoni elicits soluble factors that exert anti-microglial cell activity. Infect Immun 78:4001–4011
Hegade VS, Armstrong MJ, Smithson JAJ (2012) Hepatic schistosomiasis. QJMed 105:87
Helming L, Gordon S (2008) The molecular basis of macrophage fusion. Immunobiology 212:85–93
Hermida MD, Melo CVB, Lima IDS, Oliveira GGS, Dos-Santos WLC (2018) Histological disorganization of spleen compartments and severe leishmaniasis. Front Cell Infect Microbiol 8:1–10
Heyde S, Philipsen L, Formaglio P, Fu Y, Baars I, Höbbel G, Kleinholz CL, Seiß EA, Stettin J, Gintschel P, Dudeck A, Bousso P, Schraven B, Müller AJ (2018) CD11c-expressing Ly6C+CCR2+ monocytes constitute a reservoir for efficient Leishmania proliferation and cell-to-cell transmission. PLoS Pathog 14:e1007374
Ito T, Connett JM, Kunkel SL, Matsukawa A (2013) The linkage of innate and adaptive immune response during granulomatous development. Front Immunol 4:1–8
James DG (2000) A clinicopathological classification of granulomatous disorders. J Postgrad Med 76:457–465
Jankovic D, Kullberg MC, Dombrowicz D, Barbieri S, Caspar P, Wynn TA, Paul WE, Cheever AW, Kinet JP, Sher A (1997) Fc epsilonRI-deficient mice infected with Schistosoma mansoni mount normal Th2-type responses while displaying enhanced liver pathology. J Immunol 159:1868–1875
Janitschke K, Martínez AJ, Visvesvara GS, Schuster F (1996) Animal model Balamuthia mandrillaris CNS infection: contrast and comparison in immunodeficient and immunocompetent mice: a murine model of “Granulomatous” amebic encephalitis. J Neuropathol Exp Neurol 55:815–821
Kalra SK, Sharma P, Shyam K, Tejan N, Ghoshal U (2020) Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis. Exp Parasitol 208:107788
Kaye P, Scott P (2011) Leishmaniasis: complexity at the host–pathogen interface. Nat Rev Microbiol 9:604–615
Kaye P, Svensson M, Ato M, Maroof A, Polley R, Stager S, Zubairi S, Engwerda CR (2004) The immunopathology of experimental visceral leishmaniasis. Immunol Rev 201:239–253
Kaye PM, Beattie L (2016) Lessons from other diseases: granulomatous inflammation in leishmaniasis. Semin Imunopathol 38:249–260
Khan NA (2003) Pathogenesis of Acanthamoeba infections. Microb Pathog 34:277–285
Kinard BE, Magliocca KR, Guarner J, Delille CA, Roser SM (2016) Longstanding suppurative granulomatous inflammation of the infratemporal fossa. Oral Maxillofac Surg Cases 2:14–17
Klippe JH, Kirsten D, Andrée C (2004) Rudolf Virchow (1821-1902) and the origin of the term “granuloma”. Pneumonologie 58:449–454
Kubasta M, Dusek J, Kubastová B, Kodousek R (1965) Needle biopsy of the liver in schistosomiasis mansoni. Comparison of examination of fresh tissue and sections. Gastroenterology 49:280–286
Kurban AK, Malak JA, Farah FS, Chaglassian HT (1996) Histopathology of cutaneous leishmaniasis. Arch Dermatol 93:396–401
Lambertucci JR (2010) Acute schistosomiasis mansoni: revisited and reconsidered. Mem Inst Oswaldo Cruz 105:422–435
Lambertucci JR, Serufo JC, Gerspacher-Lara R, Rayes AA, Teixeira R, Nobre V, Antunes CM (2000) Schistosoma mansoni: assessment of morbidity before and after control. Acta Trop 77:101–108
Landa A, Navarro L, Ochoa-Sánchez A, Jiménez L (2019) Taenia solium and Taenia crassiceps: miRNomes of the larvae and effects of miR-10-5p and let-7-5p on murine peritoneal macrophages. Biosci Rep 39:BSR20190152
Lee SH, Charmoy M, Romano A, Paun A, Chaves MM, Cope FO, Ralph DA, Sacks DL (2018) Mannose receptor high, M2 dermal macrophages mediate nonhealing Leishmania major infection in a Th1 immune environment. J Exp Med 215:357–375
Lima IS, Silva JS, Almeida VA, Leal FG, Souza PA, Larageira DF, Moura-Neto JP, Fraga DBM, Freitas LAR, Dos-Santos WLC (2014) Severe clinical presentation of visceral leishmaniasis in naturally infected dogs with disruption of the splenic white pulp. PLoS One 9:e87742
Lima IS, Solcá MS, Tafuri WL, Freitas LAR, Dos-Santos WLC (2019) Assessment of histological liver alterations in dogs naturally infected with Leishmania infantum. Parasit Vectors 12:487
Lin PL, Maiello P, Gideon P, Coleman T, Cadena AM, Rodgers MA, Gregg R, O’Malley M, Tomko J, Fillmore D, Frye J, Rutledge T, DiFazio RM, Janssen C, Klein E, Andersen PL, Fortune SM, Flynn JL (2016) PET CT identifies reactivation risk in cynomolgus macaques with latent M. tuberculosis. PLoS Pathog 12:e1005739
Londoño DP, Alvarez JI, Trujillo J, Jaramillo MM, Restrepo BI (2002) The inflammatory cell infiltrates in porcine cysticercosis: immunohistochemical analysis during various stages of infection. Vet Parasitol 109:249–259
Lundy SK, Lukacs NW (2013) Chronic schistosome infection leads to modulation of granuloma formation and systemic immune suppression. Front Immunol 4:1–17
Maia C, Campino L (2018) Biomarkers associated with Leishmania infantum exposure, infection, and disease in dogs. Front Cell Infect Microbiol 8:302
Mansour L, el-Marhoumy SM, Eid MM, Gawish K (1993) A histopathological study of different clinical forms of cutaneous leishmaniasis. J Egypt Soc Parasitol 23:591–597
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16:273–307
Mariano M (1995) The experimental granuloma. A hypothesis to explain the persistence of the lesion. Rev Inst Med Trop São Paulo 37:161–176
Martínez AJ (1982) Acanthamoebiasis and immunosuppression: case report. J Neuropathol Exp Neurol 45:548–557
McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou X-N (2018) Schistosomiasis. Nat Rev Dis Primers 4(1):13. https://doi.org/10.1038/s41572-018-0013-8
Meir M, Grosfeld T, Barkan D (2018) Establishment and validation of Galleria mellonella as a novel organism to study Mycobacterium abscessus infection, pathogenesis and treatment. Antimicrob Agents Chemother 62:02539–02547
Mendonça MG, Brito MEF, Rodrigues HEG, Bandeira V, Jardim ML, Abath FGC (2004) Persistence of Leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure? J Infect Dis 189:1018–1023
Modica S, Miracco C, Cusi MG, Tordini G, Muzii VF, Iacoangeli F, Nocentini C, Ali IKM, Roy S, Cerase A, Zanelli G, De Luca A, Montagnani F (2018) Non-granulomatous cerebellar infection by Acanthamoeba spp. in an immunocompetent host. Infection 46(6):885–889
Mkupasi EM, Sikasunge CS, Ngowi HA, Leifsson PS, Johansen MV (2013) Detection of cysteine protease in Taenia solium-induced brain granulomas in naturally infected pigs. Vet Parasitol 197:360–363
Muzii VF, Iacoangeli F, Karin MAI NC, Roy S, Cerase A, Zanelli G, De Luca A, Montagnani F (2018) Non-granulomatous cerebellar infection by Acanthamoeba spp. in an immunocompetent host. Infection 46:885–889
Mohamed ARES, Karawi MAA, Yasawy MI (1990) Schistosomal colonic disease. Gut 31:439–442
Molinari JL, Tato P, Reynoso AO, Cázares JM (1990) Depressive effect of a Taenia solium cysticercus factor on cultured human lymphocytes stimulated with phytohaemagglutinin. Ann Trop Med Parasitol 84:205–208
Montoya JG, Liensenfeld O (2004) Toxoplasmosis. Lancet 363:1965–1976
Moore JWJ, Moyo D, Beattie L, Andrews PS, Timmis J, Kaye PM (2013) Functional complexity of the Leishmania granuloma and the potential of in silico modeling. Front Immunol 4:1–7
Moyo D, Beattie L, Andrews PS (2018) Macrophage transactivation for chemokine production identified as a negative regulator of granulomatous inflammation using agent-based modeling. Front Immunol 9:637
Müller AJ, Filipe-Santos O, Eberl G, Aebischer T, Späth GF, Bousso P (2012) CD4+ T cells rely on a cytokine gradient to control intracellular pathogens beyond sites of antigen presentation. Immunity 37:147–157
Murray HW (2001) Tissue granuloma structure-function in experimental visceral leishmaniasis. Int J Exp Pathol 82:249–267
Murray HW, Squires KE, Miralles CD, Stockle MY, Granger AM, Granelli-Piperno A, Bogdan C (1992) Acquired resistance and granuloma formation in experimental visceral leishmaniasis. Differential T cell and lymphokine roles in initial versus established immunity. J Immunol 148:1858–1863
Nash TE, Pretell EJ, Lescano AG, Bustos JA, Gilman RH, Gonzalez E, Garcia HH, Cysticercosis Working Group (2018) Seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol 7:1099–1105
Nathan C (2016) Macrophages’ choice: take it in or keep it out. Immunity 45:710–711
Nieto A, Domínguez-Bernal G, Orden JA, Fuente RD, Madrid-Elena N, Carríon J (2011) Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus Syrian hamster model. Vet Res 42:39
Págan AJ, Ramakrishnan L (2018) The formation and function of granulomas. Annu Rev Immunol 36:23–27
Pampiglione S, Manson-Bahr PEC, Giungi F, Giungi G, Parenti A, Trotti CG (1974) Studies on Mediterranean leishmaniasis. 2. Asymptomatic cases of visceral leishmaniasis. Trans R Soc Trop Med Hyg 68:447–453
Park MK, Hoffmann KF, Cheever AW, Amichay D, Wynn TA, Farber JM (2001) Patterns of chemokine expression in models of Schistosoma mansoni inflammation and infection reveal relationships between type 1 and type 2 responses and chemokines in vivo. Infect Immun 69:6755–6768
Pearce EJ (2005) Priming of the immune response by schistosome eggs. Parasite Immunol 27:265–270
Perez MT, Bush LM (2007) Fatal amebic encephalitis caused by Balamuthia mandrillaris in an immunocompetent host. A clinicopathological review of pathogenic free-living amebae in human hosts. Case Report. Ann Diagn Pathol 11:440–447
Pettit DAD, Williamson J, Cabral GA, Marciano-Cabral F (1996) In vitro destruction of nerve cell cultures by Acanthamoeba spp.: a transmission and scanning electron microscopy study. J Parasitol 82:769–777
Pfohl JC, Dewey CW (2005) Intracranial Toxoplasma gondii granuloma in a cat. J Feline Med Surg 7:369–374
Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceição-Silva F, Modlin RL (1993) Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest 91:1390–1395
Pisarski K (2019) The global burden of diseases of zoonotic parasitic diseases: top 5 contenders for priority consideration. Trop Med Infect Dis 4:44–52
Ramakrishnan L (2012) Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol 12:352–366
Restrepo BI, Alvarez JI, Castaño JA, Arias LF, Restrepo M, Trujillo J, Colegial CH, Teale JM (2001) Brain granulomas in neurocysticercosis patients are associated with a Th1 and Th2 profile. Infect Immun 69:4554–4560
Ridley DS (1980) A histological classification of cutaneous leishmaniasis and its geographical expression. Trans R Soc Trop Med Hyg 74:515–521
Ridley MJ, Ridley DS (1986) Monocyte recruitment, antigen degradation and localization in cutaneous leishmaniasis. Br J Exp Pathol 67:209–218
Robert-Gangneaux F, Drogoul AS, Rostan O (2012) Invariant NKT cells drive hepatic cytokinic microenvironment favoring efficient granuloma formation and early control of Leishmania donovani infection. PLoS One 7:e33413
Robinson P, Garza A, Weinstock J, Serpa JA, Goodman JC, Eckols KT, Firogary B, Tweardy DJ (2012) Substance P causes seizures in neurocysticercosis. PLoS Pathog 8:e1002489–e1002499
Rodrigues V, Cordeiro-da-Silva A, Laforge M, Silvestre R, Estaquier J (2016) Regulation of immunity during visceral Leishmania infection. Parasit Vectors 9:118
Roque AL, Jansen AM (2014) Wild and synanthropic reservoirs of Leishmania species in the Americas. Int J Parasitol Parasites Wildl 3:251–262
Saadatnia G, Golkar M (2002) A review on human toxoplasmosis. Scand J Infect Dis 44:805–814
Saeij JP, Boyle JP, Boothroyd JC (2005) Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol 21:476–481
Safaei A, Motazedian MH, Vasei M (2002) Polymerase chain reaction for diagnosis of cutaneous leishmaniasis in histologically positive, suspicious and negative skin biopsies. Dermatol 205:18–24
Salguero FJ, Garcia-Jimenez L, Lima I, Seifert K (2018) Histopathological and immunohistochemical characterisation of hepatic granulomas in Leishmania donovani-infected BALB/c mice: a time course study. Parasit Vectors 11:73
Sanchez MA, Diaz NL, Zerpa O, Negron E, Convit J, Tapia FJ (2004) Organ-specific immunity in canine visceral leishmaniasis: analysis of symptomatic and asymptomatic dogs naturally infected with Leishmania chagasi. Am J Trop Med Hyg 70:618–624
Sant’Ana JAP, Lima WG, Oliveira MR, Simões LA, Michalick MSM, Melo MN, Tafuri WL, Tafuri WL (2007) Hepatic granulomas in canine visceral leishmaniasis and clinical status. Arq Bras Med Vet Zootec 59:1137–1144
Saunders BM, Cooper AM (2000) Restraining mycobacteria role of granulomas in mycobacterial infections. Immunol Cell Biol 78:334–341
Schaeffer M, Han SJ, Chtanova T, Dooren GG, Herzmark P, Chen Y, Roysam B, Striepen B, Robey EA (2009) Dynamic imaging of T cell-parasite interactions in the brains of mice chronically infected with Toxoplasma gondii. J Immunol 182:6379–6393
Schlüter D, Löhler J, Deckert M, Hof H, Schwendemann G (1991) Toxoplasma encephalitis of immunocompetent and nude mice: immunohistochemical characterisation of Toxoplasma antigen, infiltrates and major histocompatibility complex gene products. J Neuroimmunol 31:185–198
Schlüter D, Barragan A (2019) Advances and challenges in understanding cerebral toxoplasmosis. Front Immunol 10:242–257
Schwartz C, Fallon PG (2018) Schistosoma “eggs-iting” the host: granuloma formation and egg excretion. Front Immunol 9:2492–2507
Seitzer U, Haas H, Gerdes J (2001) A human in vitro granuloma model for the investigation of multinucleated giant cell and granuloma formation. Histol Histopathol 16:645–653
Shah KK, Pritt BS, Alexander MP (2016) Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis 7:1–12
Silveira FT, Lainson R, Corbett CEP (2004) Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil-a review. Mem Inst Oswaldo Cruz 99:239–2351
Sing OP, Hasker E, Sacks D, Boelaert M, Sundar S (2014) Asymptomatic leishmania infection: a new challenge for Leishmania control. Clin Infect Dis 58:1424–1429
Souza-Lemos C, Campos SN, Teva A, Côrte-Real S, Fonseca EC, Porrozzi R, Grimaldi G Jr (2008) Dynamics of immune granuloma formation in a Leishmania braziliensis-induced self-limiting cutaneous infection in the primate Macaca mulatta. J Pathol 216:375–386
Strickland GT (1994) Gastrointestinal manifestations of schistosomiasis. Gut 35:1334–1337
Stringer JL, Marks LM, White AC Jr, Robinson P (2003) Epileptogenic activity of granulomas associated with murine cysticercosis. Exp Neurol 183:532–536
Suzuki Y, Orellana MA, Wong S, Conley FK, Remington JS (1993) Susceptibility of chronic infection with Toxoplasma gondii does not correlate with susceptibility to acute infection in mice. Infect Immun 61:2284–2288
Swe T, Baqui A, Naimg AT, Baqui T, Sherigar J, Mansour M (2016) Non-necrotizing colonic granuloma induced by schistosomiasis. J Community Hosp Intern Med Perspect 6:33114–33116
Tato P, Castro AM, Soto R, Arechavaleta F, Molinari JL (1995) Suppression of murine lymphocyte proliferation induced by a small RNA purified from the Taenia solium metacestode. Parasitol Res 81:181–187
Tenor JL, Oehlers SH, Yang JL, Tobin DM, Prefect JR (2015) Live imaging of host-parasite interactions in a zebrafish infection model reveals cryptococcal determinants of virulence and central nervous system invasion. mBio 6:e01425–e01415
Teva A, Porrozi R, Cupolillo E, Pirmez C, Oliveira-Neeto MP, Grimaldi G Jr (2003) Leishmania (Viannia) braziliensis-induced chronic granulomatous cutaneous lesions affecting the nasal mucosa in the Rhesus Monkey (Macaca Mulatta) model. Parasitology 127:437–447
Thomas M, Rao R, Kumar GN (2018) An overview of suppurative granuloma. Indian J Dermatopathol Diagn Dermatol 5:19–26
Tuon FF, Fernandes RR, Pagliari DMIS, Amato VS (2010) The expression of TLR9 in human cutaneous leishmaniasis is associated with granuloma. Parasite Immunol 32:769–772
Turk JL (1998) Encyclopedia of immunology. 2nd ed. Roitt & Delves, London, pp 1023–1026
Van den Bossche J, Laoui D, Naessens T, Smits HH, Hokke CH, Stijlemans B, Grooten J, Baetselier P, Ginderachter JAV (2015) E-cadherin expression in macrophages dampens their inflammatory responsiveness in vitro, but does not modulate M2-regulated pathologies in vivo. Sci Rep 5:12599–12610
Venkataram M, Moosa M, Devi L (2001) Histopathological spectrum in cutaneous leishmaniasis: a study in Oman. Indian J Dermatol Venereol Leprol 67:294–298
Veress B, Abdalla RE, El Hassan AM (1983) Visceral spreading depletion of thymus-dependent regions and amyloidosis in mice and hamsters infected intradermally with Leishmania isolated from Sudanese cutaneous leishmaniasis. Br J Exp Pathol 64:505–514
Visvesvara GS, Moura H, Schuster FL (2007) Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50:1–26
Voieta I, Queiroz LC, Andrade LM, Silva LCS, Fontes VF, Barbosa A Jr, Resende V, Petroianu A, Andrade Z, Antunes CM, Lambertucci JR (2010) Imaging techniques and histology in the evaluation of liver fibrosis in hepatosplenic schistosomiasis mansoni in Brazil: a comparative study. Mem Inst Oswaldo Cruz 105:414–421
Weerakoon KGAD, Gobert GN, Cai P, McManus DP (2015) Advances in the diagnosis of human schistosomiasis. Clin Microbiol Ver 28:939–967
White AC Jr (2000) Neurocysticercosis: updates on epidemiology, pathogenesis, diagnosis, and management. Annu Ver Med 51:187–206
Williams GT, Williams WJ (1983) Granulomatous inflammation-a review. J Clin Pathol 36:723–733
Williams ME, Montenegro S, Domingues AL (1994) Leucocytes of patients with Schistosoma mansoni respond with a Th2 pattern of cytokine production to mitogen or egg antigens but with a Th) pattern to worm antigens. J Infect Dis 170: 946-954.
Wilson JL, Mayr HK, Weichhart T (2019) Metabolic programming of macrophages: implications in the pathogenesis of granulomatous disease. Front Immunol 10:2265
Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA (2007) Immunopathology of schistosomiasis. Immunol Cell Biol 85:148–154
Yadav D, Ramam M (2018) Epithelioid cell granuloma. Indian J Dermatopathol Diagn Dermatol 5:7–18
Zumla A, James DG (1996) Granulomatous infections: etiology and classification. Clin Infect Dis 23:146–158
Acknowledgments
We thank the unknown referee for constructive review of the paper, Noili Demaman for English language editing, and Ana Carolina Andrade Vitor Kayano for artwork.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Ramaswamy Kalyanasundaram
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giorgio, S., Gallo-Francisco, P.H., Roque, G.A.S. et al. Granulomas in parasitic diseases: the good and the bad. Parasitol Res 119, 3165–3180 (2020). https://doi.org/10.1007/s00436-020-06841-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06841-x