Abstract
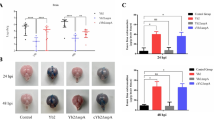
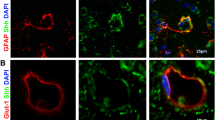
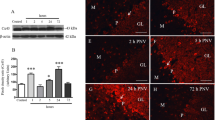
Angiostrongylus cantonensis causes a human central nervous system (CNS) infection characterized by eosinophilic meningitis or meningoencephalitis. Individuals infected with A. cantonensis exhibit unbalanced walking. The mechanism of extensive neurological impairments of hosts caused by A. cantonensis larvae remains unclear. Tight junction proteins (e.g., claudin-5 and zonula occludens-1) are the most important regulators of paracellular permeability and cellular adhesion. In a previous study, we found that increased matrix metalloproteinase-9 (MMP-9) activity may be associated with blood–CNS barrier disruption and/or the degeneration of Purkinje cells in eosinophilic meningitis caused by A. cantonensis. In the present study, the co-localization of MMP-9 and tight junction proteins on the degeneration of Purkinje cells was measured via confocal laser scanning immunofluorescence microscopy. The statistical evidence indicated that MMP-9 correlated between tight junction protein disruption and Purkinje cell degeneration at 20 days post-infection with A. cantonensis. In conclusion, Purkinje cell degeneration is highly correlated with tight junction protein disruption via the MMP-9 activation pathway.





Similar content being viewed by others
References
Ayoub AE, Cai TQ, Kaplan RA, Luo J (2003) Developmental expression of matrix metalloproteinases 2 and 9 and their potential role in the histogenesis of the cerebellar cortex. J.metalloproteinases. Brain Res 963:252–261
Balda MS, Matter K (1998) Tight junctions. J Cell Sci 111:541–547
Bednarczyk J, Lukasiuk K (2011) Tight junctions in neurological diseases. Acta Neurobiol Exp (Wars) 71:393–408
Candelario-Jalil E, Yang Y, Rosenberg GA (2009) Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 158:983–994
Chen KM, Lee HH, Lu KH, Tseng YK, Hsu LS, Chou HL, Lai SC (2004) Association of matrix metalloproteinase-9 and Purkinje cell degeneration in mouse cerebellum caused by Angiostrongylus cantonensis. Int J Parasitol 34:1147–1156
Chen KM, Liu JY, Lai SC, Hsu LS, Lee HH (2006) Association of plasminogen activators and matrix metalloproteinase-9 proteolytic cascade with blood-CNS barrier damage of angiostrongyliasis. Int J Exp Pathol 87:113–119
Chen AC, Shyu LY, Lin YC, Chen KM, Lai SC (2019) Proteasome serves as pivotal regulator in Angiostrongylus cantonensis-induced eosinophilic meningoencephalitis. PLoS One 14:e0220503
Chiu PS, Lai SC (2013) Matrix metalloproteinase-9 leads to claudin-5 degradation via the NF-κB pathway in BALB/c mice with eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis. PLoS One 8:e53370
Deb S, Gottschall PE (1996) Increased production of matrix metalloproteinases in enriched astrocyte and mixed hippocampal cultures treated with beta-amyloid peptides. J Neurochem 66:1641–1647
Durack DT, Sumi SM, Klebanoff SJ (1979) Neurotoxicity of human eosinophils. Proc Natl Acad Sci U S A 76:1443–1447
Fredens K, Dahl R, Venge P (1982) The Gordon phenomenon induced by the eosinophil cationic protein and eosinophil protein X. J. Allergy Clin Immunol 70:361–366
Gardiner CH, Wells S, Gutter AE, Fitzgerald L, Anderson DC, Harris RK, Nichols DK (1990) Eosinophilic meningoencephalitis due to Angiostrongylus cantonensis as the cause of death in captive non-human primates. Am J Trop Med Hyg 42:70–74
Gottschall PE (1996) Beta-amyloid induction of gelatinase B secretion in cultured microglia: inhibition by dexamethasone and indomethacin. Neuroreport 7:3077–3080
Gurney KJ, Estrada EY, Rosenberg GA (2006) Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis 23:87–96
Hartsock A, Nelson WJ (2008) Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778:660–669
Hawkins KE, DeMars KM, Yang C, Rosenberg GA, Candelario-Jalil E (2013) Fluorometric immunocapture assay for the specific measurement of matrix metalloproteinase-9 activity in biological samples: application to brain and plasma from rats with ischemic stroke. Mol Brain 6:14. https://doi.org/10.1186/1756-6606-6-14
Hou RF, Tu WC, Lee HH, Chen KM, Chou HL, Lai SC (2004) Elevation of plasminogen activators in cerebrospinal fluid of mice with eosinophilic meningitis caused by Angiostrongylus cantonensis. Int J Parasitol 34:1355–1364
Hsu WY, Chen JY, Chien CT, Chi CS, Han NT (1990) Eosinophilic meningitis caused by Angiostrongylus cantonensis. Pediatr Infect Dis J 9:443–445
Ishihara H, Kubota H, Lindberg RL, Leppert D, Gloor SM, Errede M, Virgintino D, Fontana A, Yonekawa Y, Frei K (2008) Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J Neuropathol Exp Neurol 67:435–448
Lai SC, Twu JJ, Jiang ST, Hsu JD, Chen KM, Chiaing HC, Wang CJ, Tseng CK, Shyu LY, Lee HH (2004) Induction of matrix metalloproteinase-9 in murine eosinophilic meningitis caused by Angiostrongylus cantonensis. Ann Trop Med Parasitol 98:715–724
Lakhan SE, Kirchgessner A, Tepper D, Leonard A (2013) Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol 4:32. https://doi.org/10.3389/fneur.2013.00032 eCollection 2013
Lee HH, Chou HL, Chen KM, Lai SC (2004) Association of matrix metalloproteinase-9 in eosinophilic meningitis of BALB/c mice caused by Angiostrongylus cantonensis. Parasitol Res 94:321–328
Leib SL, Clements JM, Lindberg RL, Heimgartner C, Loeffler JM, Pfister LA, Täuber MG, Leppert D (2001) Inhibition of matrix metalloproteinases and tumour necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain 124:1734–1742
Leppert D, Waubant E, Galardy R, Bunnett NW, Hauser SL (1995) T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol 154:4379–4389
Liao G, Wang Z, Lee E, Moreno S, Abuelnasr O, Baudry M, Bi X (2015) Enhanced expression of matrix metalloproteinase-12 contributes to Npc1 deficiency-induced axonal degeneration. Exp Neurol 269:67–74
Lin KY, Chen KM, Lan KP, Lee HH, Lai SC (2010) Alterations of myelin proteins in inflammatory demyelination of BALB/c mice caused by Angiostrongylus cantonensis. Vet Parasitol 171:74–80
Masure S, Proost P, van Damme J, Opdenakker G (1991) Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur J Biochem 198:391–398
Morocoima A, Socorro G, Avila R, Hernández A, Merchán S, Ortiz D, Primavera G, Chique J, Herrera L, Urdaneta-Morales S (2012) Trypanosoma cruzi: experimental parasitism in the central nervous system of albino mice. Parasitol Res 111:2099–2107
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases: a mini-review. J Biol Chem 274:21491–21494
Nico B, Corsi P, Ria R, Crivellato E, Vacca A, Roccaro AM, Mangieri D, Ribatti D, Roncali L (2006) Increased matrix-metalloproteinase-2 and matrix-metalloproteinase-9 expression in the brain of dystrophic mdx mouse. Neuroscience 140:835–848
Nielsen BS, Timshel S, Kjeldsen L, Sehested M, Pyke C, Borregaard N, Danø K (1996) 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int J Cancer 65:57–62
Niessen C (2007) Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol 127:2525–2532
Nishimura K, Hung T (1997) Current views on geographic distribution and modes of infection of neurohelminthic diseases. J Neurol Sci 145:5–14
Rosenberg GA (2009) Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8:205–216
Spring K (1998) Routes and mechanism of fluid transport by epithelia. Annu Rev Physiol 60:105–119
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Tsukita S, Furuse M, Itoh M (1999) Structural and signaling molecules come together at tight junctions. Curr Opin Cell Biol 11:628–633
Vaillant C, Didier-Bazès M, Hutter A, Belin MF, Thomasset N (1999) Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J Neurosci 19:4994–5004
Wächter C, Eiden LE, Naumann N, Depboylu C, Weihe E (2016) Loss of cerebellar neurons in the progression of lentiviral disease: effects of CNS-permeant antiretroviral therapy. J Neuroinflammation 13:272. https://doi.org/10.1186/s12974-016-0726-0
Welgus HG, Campbell EJ, Cury JD, Eisen AZ, Senior RM, Wilhelm SM, Goldberg GI (1990) Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest 86:1496–1502
Wright JW, Masino AJ, Reichert JR, Turner GD, Meighan SE, Meighan PC, Harding JW (2005) Ethanol-induced impairment of spatial memory and brain matrix. J Comp Neurol 481:403–415
Yang Y, Rosenberg GA (2011) MMP-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia. Methods Mol Biol 762:333–345
Yong VW, Power C, Forsyth P, Edwards DR (2001) Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci 2:502–511
Yoshimura K (1993) Mechanism of parasite killing by eosinophils in parasitic infections. Nippon Rinsho 51:657–663
Yoshimura K, Sugaya H, Ishida K (1994) The role of eosinophils in Angiostrongylus cantonensis infection. Parasitol Today 10:231–233
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The manuscript does not contain clinical or patient data.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: David Bruce Conn
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lai, SC., Lu, CY., Shyu, LY. et al. Angiostrongylus cantonensis infection induces MMP-9 and causes tight junction protein disruption associated with Purkinje cell degeneration. Parasitol Res 119, 3433–3441 (2020). https://doi.org/10.1007/s00436-020-06840-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06840-y