Abstract
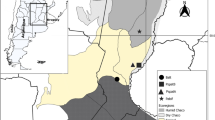
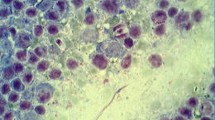
Amphibians are among the most threatened vertebrate groups in the world, and the main causes include climate change, habitat destruction, and emerging diseases. Herein, we investigated the occurrence and characterized molecularly Apicomplexa in anurans from southeastern Brazil. Forty individuals from seven anuran species were sampled in São Paulo state. In the molecular analyses, one Leptodactylus latrans and one Rhinella diptycha were positive in PCR assays for species of Hepatozoon. Two L. latrans were also positive for coccidian infections (Lankesterella sp. and an unidentified coccidian species). Phylogenetic analysis based on 18S rDNA clustered the sequences detected in anurans from the present study with Hepatozoon spp. detected in reptiles and other anurans from Brazil, albeit they were separate from Hepatozoon haplotypes detected in frogs from Africa and North America. Our study showed, for the first time, the molecular detection of Lankesterella sp. and another coccidian in L. latrans. Additionally, co-infection by different species of Hepatozoon haplotypes and an unidentified coccidian in anurans from Brazil was documented.


Similar content being viewed by others
References
Abella-Medrano CA, Ibáñez-Bernal S, MacGregor-Fors I, Santiago-Alarcon D (2015) Spatiotemporal variation of mosquito diversity (Diptera: Culicidae) at places with different land-use types within a neotropical montane cloud forest matrix. Parasit Vectors 8:1–11. https://doi.org/10.1186/s13071-015-1086-9
André MR, Adania CH, Teixeira RHF, Vargas GH, Falcade M, Sousa L, Salles AR, Allegretti SM, Felippe PAN, Machado RZ (2010) Molecular detection of Hepatozoon spp. in Brazilian and exotic wild carnivores. Vet Parasitol 173:134–138. https://doi.org/10.1016/j.vetpar.2010.06.014
Barta JR, Desser SS (1984) Blood parasites of amphibians from Algonquin Park, Ontario. J Wildl Dis 20:180–189. https://doi.org/10.7589/0090-3558-20.3.180
Barta JR, Ogedengbe JD, Martin DS, Smith TG (2012) Phylogenetic position of the adeleorinid coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rDNA sequences. J Eukaryot Microbiol 59:171–180
Becker CG, Fonseca CR, Haddad CFB, Prado PI (2010) Habitat split as a cause of local population declines of amphibians with aquatic larvae: Contributed paper. Conserv Biol 24:287–294. https://doi.org/10.1111/j.1523-1739.2009.01324.x
Benson DA, Karsch-mizrachi I, Lipman DJ et al (2000) GenBank 28:15–18
Bouer A, André MR, Gonçalves LR, Luzzi MC, Oliveira JP, Rodrigues AC, Varani AM, Miranda VFO, Perles L, Werther K, Machado RZ (2017) Hepatozoon caimani in Caiman crocodilus yacare (Crocodylia, Alligatoridae) from North Pantanal, Brazil. Brazilian J Vet Parasitol 26:352–358
Carini (1931) Eimeria leptodactyli n. sp. encontrada no intestino do Leptodactylus ocellatus. Arq Biol 15:256
da Silva MRL, Fornazari F, Martins TF, Hippólito AG, Rolim LS, Bisca JM, Teixeira CR, LH O’D (2018) A survey of hemoparasites and ectoparasites in Nasua nasua (Linnaeus, 1766) with a redescription of Hepatozoon procyonis (Richards, 1961) based on morphological and molecular data. Parasitol Res 117:2159–2169. https://doi.org/10.1007/s00436-018-5903-x
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and high-performance computing Europe PMC Funders Group. Nat Methods 9:772. https://doi.org/10.1038/nmeth.2109.jModelTest
Davies AJ, Johnston MRL (2000) The biology of some intraerythrocytic parasites of fishes, amphibia and reptiles. Adv Parasitol 45:1–107. https://doi.org/10.1016/s0065-308x(00)45003-7
de Miranda RL, O’Dwyer LH, de Castro JR, Metzger B, Rubini AS, Mundim AV, Eyal O, Talmi-Frank D, Cury MC, Baneth G (2014) Prevalence and molecular characterization of Hepatozoon canis in dogs from urban and rural areas in Southeast Brazil. Res Vet Sci 97:325–328. https://doi.org/10.1016/j.rvsc.2014.06.015
de Sousa KCM, Fernandes MP, Herrera HM, Benevenute JL, Santos FM, Rocha FL, Barreto WT, Macedo GC, Campos JB, Martins TF, de Andrade Pinto PC, Battesti DB, Piranda EM, Cançado PH, Machado RZ, André MR (2017) Molecular detection of Hepatozoon spp. in domestic dogs and wild mammals in southern Pantanal, Brazil with implications in the transmission route. Vet Parasitol 237:37–46. https://doi.org/10.1016/j.vetpar.2017.02.023
Densmore CL, Green DE (2007) Diseases of amphibians. ILAR J 48:235–254. https://doi.org/10.1093/ilar.48.3.235
Desser SS (2001) The Blood Parasites of Anurans from Costa Rica with Reflections on the Taxonomy of Their Trypanosomes. J Parasitol 87:152–160. https://doi.org/10.2307/3285193
Duszynski DW, Bolek MG, Upton SJ (2007) Coccidia (Apicomplexa: Eimeriidae) of amphibians of the world. Zootaxa 1667:1–77
Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using Phred. I. Acuracy Assessement. Genome Res 8:1975–1985
Ewing B, Green P (1998) Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res 8:186–194. https://doi.org/10.1101/gr.8.3.186
Faggioni GP, Souza FL, Uetanabaro M, Landgref Filho P, Prado CPA (2017) Diet of Leptodactylus bufonius Boulenger, 1894, in the Brazilian Chaco. Herpetozoa 30:72–76
Ferreira JIGS, Costa AP, Ramirez D, Roldan JAM , Saraiva D, Founier GFRS, Sue A, Zambelli ER , Minervino AHH, Verdade VK, Gennari SM, Marcili A (2015) Anuran trypanosomes: phylogenetic evidence for new clades in Brazil. Syst Parasitol 91(1):63–70
Frost DR (2020) Amphibian Species of the World: an Online Reference. Version 6.0 (12 February 2020). Electronic Database accessible at http://research.amnh.org/herpetology/amphibia/index.html. American Museum of Natural History, New York, USA. https://doi.org/10.5531/db.vz.0001.
Harris DJ, Graciá E, Jorge F, Maia JPMC, Perera A, Carretero MA, Giménez A (2013) Molecular Detection of Hemolivia (Apicomplexa: Haemogregarinidae) from Ticks of North African Testudo graeca (Testudines: Testudinidae) and an Estimation of Their Phylogenetic Relationships Using 18S rRNA Sequences. Comparative Parasit 80:292–296
Harkness LM, Drohan AE, Dickson CM, Smith TG (2010) Experimental Transmission of Hepatozoon clamatae (Apicomplexa: Adeleida) to the Wood Frog, Rana sylvatica, and to the Mosquito Culex pipiens. J Parasitol 96:434–436. https://doi.org/10.1645/ge-2317.1
Jenkinson TS, Betancourt Román CM, Lambertini C, Valencia-Aguilar A, Rodriguez D, Nunes-de-Almeida CHL, Ruggeri J, Belasen AM, da Silva Leite D, Zamudio KR, Longcore JE, Toledo LF, James TY (2016) Amphibian-killing chytrid in Brazil comprises both locally endemic and globally expanding populations. Mol Ecol 25(13):2978–2996
Katoh K, Rozewicki J, Yamada KD (2017) MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. https://doi.org/10.1093/bib/bbx108
Korzh AP, Zadorozhnyaya VY (2013) The biological characteristic of representatives of the genus Hepatozoon (Apicomplexa, Adeleorina) in the marsh frog (Pelophylax ridibundus) separate populations of Zaporozhzhya region. Vestnik Zoologii 42:34–39
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549
Lainson R, Paperna I (1995) Light and electron microscope study of a Lankesterella petiti n. sp., (Apicomplexa: Lankesterellidae) infecting Bufo marinus (Amphibia: Anura) in Pará, North Brazil. Parasite 2:307–313. https://doi.org/10.1051/parasite/1995023307
Lainson R, Paperna I, Naiff RD (2003) Development of Hepatozoon caimani (Carini, 1909) Pessôa, De Biasi & De Souza, 1972 in the Caiman Caiman c. crocodilus, the Frog Rana catesbeiana and the Mosquito Culex fatigans. Mem Inst Oswaldo Cruz 98:103–113. https://doi.org/10.1590/S0074-02762003000100014
Lau AOT, Cereceres K, Palmer GH, Fretwell DL, Pedroni MJ, Mosqueda J, McElwain TF (2010) Genotypic diversity of merozoite surface antigen 1 of Babesia bovis within an endemic population. Mol Biochem Parasitol 172:107–112. https://doi.org/10.1016/j.molbiopara.2010.03.017
Leal DDM, Dreyer CS, da Silva RJ, Ribolla PE, Paduan KS, Biachi I, O’Dwyer LH (2015) Characterization of Hepatozoon spp. in Leptodactylus chaquensis and Leptodactylus podicipinus from two regions of the Pantanal, state of Mato Grosso do Sul, Brazil. Parasitol Res 114:1541–1549. https://doi.org/10.1007/s00436-015-4338-x
Léveillé AN, Baneth G, Barta JR (2019) Next generation sequencing from Hepatozoon canis (Apicomplexa: Coccidia: Adeleorina): complete apicoplast genome and multiple mitochondrion-associated sequences. I J Parasitol 49(5):375–387
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25(11):1451–1452
França LF, Facure K, Giareta AA (2004) Trophic and Spatial Niches of two Large-sized Species Leptodactylus (Anura) in Southeastern Brazil. Stud Neotropical Fauna Environ 39:243–248
Macale D, Vignoli L, Carpaneto GM (2008) Food selection strategy during the reproductive period in three syntopic hylid species from a subtropical wetland of north-east Argentina. Herpetol J 18:49–58
Maia JPMC, Harris DJ, Perera A (2011) Molecular survey of Hepatozoon species in lizards from North Africa. J Parasitol 97:513–517
McDiarmid RW (1994) Preparing amphibians as scientific specimens. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS (eds) Measuring and Monitoring Biological Diversity. Standard Methods for Amphibians, p.289-297. Smithsonian, Washington
Miller WW (1908) Hepatozoon perniciosum n.g., n. sp., a haemogregarine pathogenic for white rats; with a brief description of the sexual cycle in the intermediate host, a mite (Laelaps echidninus). Bull Hyg Lab Washingt 46:51–123
Murphey FJPP, Burbutis ADFB (1967) Bionomics of Culex salinarius Coquillett. II. Host acceptance and feeding by adult females of C. salinarius and other mosquito species. Mosq News 27:366–374
Netherlands EC, Cook CA, Smit NJ (2014a) Hepatozoon species (Adeleorina: Hepatozoidae) of African bufonids, with morphological description and molecular diagnosis of Hepatozoon ixoxo sp. nov. parasitising three Amietophrynus species (Anura: Bufonidae). Parasites Vectors 7:552.
Netherlands EC, Cook CA, Smit NJ (2014b) Hepatozoon species (Adeleorina: Hepatozoidae) of African bufonids, with morphological description and molecular diagnosis of Hepatozoon ixoxo sp. nov. parasitising three Amietophrynus species (Anura: Bufonidae). Parasit Vectors 7:552–564
Netherlands EC, Cook CA, Du Preez LH et al (2018) Monophyly of the species of Hepatozoon (Adeleorina: Hepatozoidae) parasitizing (African) anurans, with the description of three new species from hyperoliid frogs in South Africa. Parasitology 145:1039–1050. https://doi.org/10.1017/S003118201700213X
Netherlands EC, Cook CA, Kruger DJD, du Preez LH, Smit NJ (2015) Biodiversity of frog haemoparasites from sub-tropical northern KwaZulu-Natal, South Africa. Int J Parasitol Parasites Wildl 4:135–141. https://doi.org/10.1016/j.ijppaw.2015.01.003
Palumbi S, Martin A, Romano S, McMillan WO, Stice L, Grabowski G (1991) The Simple Fool’s Guide To PCR. Department of Zoology and Kewalo Marine Laboratory, Universiti of Hawaii, Honolulu
Palumbi S, Martin A, Romano S, McMillan WO, Stice L, Grabowski G (2002) The Simple Fool’s Guide To PCR, ver. 2. University of Hawaii, Honolulu.
Paperna I, Lainson R (1995) Life history and ultrastructure of Eimeria bufomarini n.sp. (Apicomplexa: Eimeriidae) of the giant toad, Bufo marinus (Amphibia: Anura) from Amazon Brazil. Parasite 2:141–148
Perkins SL, Keller AK (2001) Phylogeny of Nuclear Small Subunit rRNA Genes of Hemogregarines Amplified with Specific Primers. J Parasitol 87:870–876. https://doi.org/10.2307/3285147
Pimenta B, Costa D, Murta-Fonseca R, Pezzuti TL, PezzutI TL (2014) Anfíbios: Alvorada de Minas, Conceição do Mato Dentro, Dom Joaquim, Minas Gerais, 1st edn. Belo Horizonte, Bicho do Mato, 196p
Pinto C, Valim AR (1926) Estudos sobre coccideas. Bol do Inst Bras Sci 2:216–219
Posada D, Buckley TR, Thorne J (2004) Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol 53:793–808
Prado CPA (2003) Leptodactylus chaquensis (NCN), Pseudis paradoxa (Paradox frog), and Phrynohyas venulosa (Veined Treefrog). Predation. Herpetol Rev 34:231–232
Readel AM, Goldberg TL, (2010) Blood parasites of frogs from an equatorial African montane forest in western Uganda. J Parasitol 96(2):448–450
Sambrook J, Fritsch EF, Maniatis T (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sanger F, Nicklen S, CAR (1997) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci United States Am 74:5463–5467. https://doi.org/10.1097/00006250-199004001-00013
Segalla MV, Caramaschi U, Cruz CAG, Grant T, Haddad CFB, Garcia PCA, Berneck BVM, Langone JA (2016) Brazilian amphibians: list of species. Herpetologia Brasileira 5:34–46
Smith TG (1996) The genus Hepatozoon (Apicomplexa: Adeleina). J Parasitol 82(4):565
Stover BC, Müller MK (2010) TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics 11:1–9. https://doi.org/10.1186/1471-2105-11-7
Telford SR, Norton TM, Moler PE, Jensen JB (2009) A New Haemogregarina Species of the Alligator Snapping Turtle, Macrochelys temminckii (Testudines: Chelydridae), in Georgia and Florida that Produces Macromeronts in Circulating Erythrocytes. J Parasitol 95:208–214. https://doi.org/10.1645/ge-1696.1
Smith T, SS, Dresser DM (1994) The development of Hepatozoon sipedon sp. nov. (Apicomplexa: Adeleina: Hepatozoidae) in its natural host, the Northern water snake (Nerodia sipedon sipedon), in the culicine vectors Culex pipiens and C. territans, and in an intermediate host, the Northern. Parasitol Res 80:559–568
Toledo LF, Ribeiro RS, Haddad CFB (2007) Anurans as prey: an exploratory analysis and size relationships between predators and their prey. J Zool 271:170–177
Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232–W235. https://doi.org/10.1093/nar/gkw256
Uetanabaro M, Prado CPA, Rodrigues DJ, Gordo ZCM (2008) Field Guide to the Anurans of the Pantanal and Surrounding Cerrados. Mato Grosso do Sul, Brazil
Ujvari B, Madsen T, Olsson M (2004) High prevalence of Hepatozoon spp. (Apicomplexa, Hepatozoidae) infection in water pythons (Liasis fuscus) from tropical Australia. J Parasitol 90(3):670–672
Viana LA, Soares P, Silva JE, Paiva F, Coutinho ME (2012) Anurans as paratenic hosts in the transmission of Hepatozoon caimani to caimans Caiman yacare and Caiman latirostris. Parasitol Res 110:883–886. https://doi.org/10.1007/s00436-011-2570-6
Vredenburg VT, Roland AK, Tate ST, Cheryl JB (2010) Dynamics of an emerging disease drive large-scale amphibian populations extinctions. PNAS 107:9689–9694
Wake DB, Vredenburg VT (2008) Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Nat Acad Sci 105:11466–11473
Wake DB, Vredenburg VT (2009) Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Light Evol 2:27–44. https://doi.org/10.17226/12501
Funding
The authors are thankful to FAPESP (São Paulo Research Foundation—Process 2018/02753-0) for the financial support and CNPq for the Productivity Grant granted to the MRA (CNPq Process No. 302420/2017-7).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Daniel de Abreu Reis Ferreira, Cynthia Peralta de Almeida Prado, and Lívia Perles. The first draft of the manuscript was written by Daniel de Abreu Reis Ferreira, Lívia Perles, and Marcos Rogério André and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures were approved by the Chico Mendes Institute for Biodiversity Conservation (ICMBio/SISBIO) (license # 19269-1) and by the Ethics Committee on Animal Use (CEUA) of the Universidade Estadual Paulista Júlio de Mesquita Filho (CEUA FCAV # 017379/17). All experiments were conducted in accordance with SISBIO, CEUA FCAV, and CONCEA (National Council for Animal Experimentation Control) guidelines.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Sequences were deposited in GenBank under submission numbers MK508984–MK508989 and MK503642–MK503648.
Additional information
Section Editor: Panagiotis Karanis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 56 kb)
Rights and permissions
About this article
Cite this article
de Abreu Reis Ferreira, D., Perles, L., Machado, R.Z. et al. Molecular detection of Apicomplexan hemoparasites in anurans from Brazil. Parasitol Res 119, 3469–3479 (2020). https://doi.org/10.1007/s00436-020-06835-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06835-9