Abstract
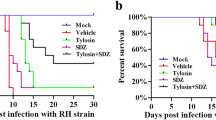
Phosphoinositide-dependent phospholipase-C (PI-PLC) triggers the calcium signaling pathway which plays an important role in dense granule and microneme secretion and pathogenesis of Toxoplasma gondii (T. gondii). There are limited data about the effects of phospholipid analogues against T. gondii. The current study assessed the effect of edelfosine, as a phospholipid analogue, on GRA1 and MIC3 expressions using in vitro and in vivo models of acute toxoplasmosis. Infected Vero cells were treated by edelfosine in two subgroups: 24 h following the cell infection and treatment at the same time of cell infection. Animal study was performed on forty mice in four groups including non-infected, infected untreated, infected edelfosine-treated, and infected pyrimethamine-treated. Gene and protein expression analyses were done using quantitative real-time PCR and western blot, respectively. Edelfosine significantly reduced the GRA1 (P < 0.01) and MIC3 (P < 0.01) mRNA and protein expressions in 24 h following the cell infection and at the same time of cell infection groups. In vivo study showed that the edelfosine significantly reduced the GRA1 expression in eye, and MIC3 expression in brain and liver. Moreover, the edelfosine-treated infected mice had significant higher survival rate compared with uninfected mice. The reducing effect of edelfosine on GRA1 and MIC3 mRNA and protein levels 24 h following the cell infection was more than treatment at the same time of cell infection group. Moreover, the effect of edelfosine on GRA1 and MIC3 expression in animal tissues was variable. These data showed that the edelfosine may decrease the T. gondii excretory/secretory antigens through inhibition of PI-PLC.







Similar content being viewed by others
References
Azzouz S, Maache M, Garcia RG, Osuna A (2005) Leishmanicidal activity of edelfosine, miltefosine and ilmofosine. Basic Clin Pharmacol Toxicol 96(1):60–65
Carruthers VB, Moreno SN, Sibley DL (1999) Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem J 342(2):379–386
Carruthers VB, Sibley LD (1997) Sequential protein secretion from three distinct organelles of toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol 73(2):114–123
Carruthers VB, Sibley LD (1999) Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol Microbiol 31(2):421–428
Charif H, Darcy F, Torpier G, Cesbron-Delauw MF, Capron A (1990) Toxoplasma gondii: characterization and localization of antigens secreted from tachyzoites. Exp Parasitol 71(1):114–124
Chaturvedi S, Qi H, Coleman D, Rodriguez A, Hanson PI, Striepen B, Roos DS, Joiner KA (1999) Constitutive calcium-independent release of Toxoplasma gondii dense granules occurs through the NSF/SNAP/SNARE/Rab machinery. J Biol Chem 274(4):2424–2431
Croft SL, Neal RA, Thornton EA, Herrmann DB (1993) Antileishmanial activity of the ether phospholipid ilmofosine. Trans R Soc Trop Med Hyg 87(2):217–219
Croft SL, Seifert K, Duchêne M (2003) Antiprotozoal activities of phospholipid analogues. Mol Biochem Parasitol 126(2):165–172
Croft SL, Snowdon D, Yardley V (1996) The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J Antimicrob Chemother 38(6):1041–1047
Eissa MM, Barakat AM, Amer EI, Younis LK (2015) Could miltefosine be used as a therapy for toxoplasmosis? Exp Parasitol 157:12–22
Escobar P, Matu S, Marques C, Croft SL (2002) Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH3 (edelfosine) and amphotericin B. Acta Trop 81(2):151–157
Fang J, Marchesini N, Moreno SN (2006) A Toxoplasma gondii phosphoinositide phospholipase C (TgPI-PLC) with high affinity for phosphatidylinositol. Biochem J 394(2):417–425
Garrison E, Treeck M, Ehret E, Butz H, Garbuz T, Oswald BP, Settles M, Boothroyd J, Arrizabalaga G (2012) A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma. PLoS Pathog 8(11):e1003049
Harnett W, Harnett MM (1998) Heterotrimeric guanine nucleotide-binding proteins in eukaryotic parasites. Parasitol Today 14(1):27–31
Heesbeen EC, Verdonck LF, Staal GE, Rijksen G (1994) Protein kinase C is not involved in the cytotoxic action of 1-octadecyl-2-O-methyl-sn-glycerol-3-phosphocholine in HL-60 and K562 cells. Biochem Pharmacol 47(9):1481–1488
Johnson AM, Mcdonald PJ, Nech SH (1979) Kinetics of the growth of Toxoplasma gondii (RH strain) in mice. Int J Parasitol 9:55–56
Johnson SM, Murphy RC, Geiger JA, DeRocher AE, Zhang Z, Ojo KK, Larson ET, Perera BG, Dale EJ, He P, Reid MC, Fox AM, Mueller NR, Merritt EA, Fan E, Parsons M, van Voorhis W, Maly DJ (2012) Development of Toxoplasma gondii calcium-dependent protein kinase 1 (Tg CDPK1) inhibitors with potent anti-toxoplasma activity. J Med Chem 55(5):2416–2426
Kieschnick H, Wakefield T, Narducci CA, Beckers C (2001) Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J Biol Chem 276(15):12369–12377
Konstantinov SM, Eibl H, Berger MR (1997) Alkylphosphocholines induce apoptosis in HL-60 and U-937 leukemic cells. Cancer Chemother Pharmacol 41(3):210–216
Liendo A, Joiner KA (2000) Toxoplasma gondii: conserved protein machinery in an unusual secretory pathway? Microbes Infect 2(2):137–144
Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD (2010) Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 465(7296):359–362
Lourido S, Zhang C, Lopez MS, Tang K, Barks J, Wang Q, Wildman SA, Shokat KM, Sibley LD (2013) Optimizing small molecule inhibitors of calcium-dependent protein kinase 1 to prevent infection by Toxoplasma gondii. J Med Chem 56(7):3068–3077
Lovett JL, Marchesini N, Moreno SN, Sibley LD (2002) Toxoplasma gondii microneme secretion involves intracellular Ca2+ release from inositol 1, 4, 5-triphosphate (IP3)/ryanodine-sensitive stores. J Biol Chem 277(29):25870–25876
Lovett JL, Sibley LD (2003) Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J Cell Sci 116(14):3009–3016
Lux H, Heise N, Klenner T, Hart D, Opperdoes FR (2000) Ether–lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether–lipid analogues in Leishmania. Mol Biochem Parasitol 111(1):1–4
Malaquias AT, Oliveira MM (1999) Phospholipid signalling pathways in Trypanosoma cruzi growth control. Acta Trop 73(2):93–108
McCoy JM, Whitehead L, van Dooren GG, Tonkin CJ (2013) Correction: TgCDPK3 regulates calcium-dependent egress of Toxoplasma gondii from host cells. PLoS Pathog 9(1)
Mollinedo F, Fernández-Luna JL, Gajate C, Martín-Martín B, Benito A, Martínez-Dalmau R, Modolell M (1997) Selective induction of apoptosis in cancer cells by the ether lipid ET-18-OCH3 (Edelfosine): molecular structure requirements, cellular uptake, and protection by Bcl-2 and Bcl-XL. Cancer Res 57(7):1320–1328
Montazeri M, Ebrahimzadeh MA, Ahmadpour E, Sharif M, Sarvi S, Daryani A (2016) Evaluation of propranolol effect on experimental acute and chronic toxoplasmosis using quantitative PCR. Antimicrob Agents Chemother 60(12):7128–7133
Montazeri M, Mehrzadi S, Sharif M, Sarvi S, Tanzifi A, Aghayan SA, Daryani A (2018) Drug resistance in toxoplasma gondii. Front Microbiol 9
Moudy R, Manning TJ, Beckers CJ (2001) The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J Biol Chem 276:41492–41501
Pace DA, McKnight CA, Liu J, Jimenez V, Moreno SN (2014) Calcium entry in toxoplasma gondii and its enhancing effect of invasion-linked traits. J Biol Chem 289(28):19637–19647
Moreno SNJ, Miranda K, Fang J, Rohloff P, de Souza W (2007) Calcium storage and homeostasis in Toxoplasma gondii. In: Weiss LM, Kim K (eds) Toxoplasma gondii, the model apicomplexan, perspectives and methods, 1st edn. Elsevier, pp 245–263
Saadatnia G, Haj Ghani H, Khoo BY, Maimunah A, Noordin R (2010) Research note optimization of toxoplasma gondii cultivation in VERO cell line. Trop Biomed 27:125–130
Seifert K, Duchêne M, Wernsdorfer WH, Kollaritsch H, Scheiner O, Wiedermann G, Hottkowitz T, Eibl H (2001) Effects of miltefosine and other alkylphosphocholines on human intestinal parasiteEntamoeba histolytica. Antimicrob Agents Chemother 45(5):1505–1510
Sidik SM, Triana MA, Paul AS, El Bakkouri M, Hackett CG, Tran F et al (2016) Using a genetically encoded sensor to identify inhibitors of toxoplasma gondii Ca2+ signalling. J Biol Chem:Jbc–M115
Sorrentino V, Barone V, Rossi D (2000) Intracellular Ca2+ release channels in evolution. Curr Opin Genet Dev 10(6):662–667
Soto J, Toledo J, Gutierrez P, Nicholls RS, Padilla J, Engel J, Fischer C, Voss A, Berman J (2001) Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin Infect Dis 33(7):e57–e61
Sundar S, Makharia A, More DK, Agrawal G, Voss A, Fischer C, Bachmann P, Murray HW (2000) Short-course of oral miltefosine for treatment of visceral leishmaniasis. Clin Infect Dis 31(4):1110–1113
Überall F, Oberhuber H, Maly K, Zaknun J, Demuth L, Grunicke HH (1991) Hexadecylphosphocholine inhibits inositol phosphate formation and protein kinase C activity. Cancer Res 51(3):807–812
van Haasteren G, Li S, Muda M, Susini S, Schlegel W (1999) Calcium signalling and gene expression. J Receptors Signal Transduct 19(1–4):481–492
Walochnik J, Duchêne M, Seifert K, Obwaller A, Hottkowitz T, Wiedermann G, Eibl H, Aspöck H (2002) Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob Agents Chemother 46(3):695–701
Wieder T, Orfanos CE, Geilen CC (1998) Induction of ceramide-mediated apoptosis by the anticancer phospholipid analog, hexadecylphosphocholine. J Biol Chem 273(18):11025–11031
Acknowledgments
We acknowledge the support by the Research Foundation of the Mazandaran University of Medical Sciences. Authors have a special appreciation to Dr. Amir Azimian for the primer design.
Funding
This work was supported financially from the vice chancellor for research at Mazandaran University of Medical Sciences (No. 1242).
Author information
Authors and Affiliations
Contributions
AT performed the cellular, animal, laboratory, and molecular testing. AK designed the study and supervised the molecular testing. SE advised selection of concentrations in cellular and animal studies. SS and MS supervised the animal study. MM collaborated in cellular testing. MM collaborated in final manuscript edition. AD designed and supervised the laboratory testing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
This study was carried out in accordance with the recommendations of Ethics commission of the Mazandaran University of Medical Sciences. The protocol was approved by the ethics committee of Mazandaran University of Medical Sciences (Project ethic number: IR.MAZUMS.REC.1397.1242).
Additional information
Section Editor: Xing-Quan Zhu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanzifi, A., Khoshi, A., Emami, S. et al. The effect of edelfosine on GRA1 and MIC3 expressions in acute toxoplasmosis. Parasitol Res 119, 1371–1380 (2020). https://doi.org/10.1007/s00436-020-06601-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06601-x