Abstract
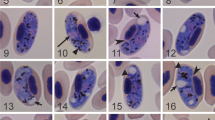
The Bellinger River snapping turtle (Myuchelys georgesi) is endemic to Australia and is confined to a highly restricted distribution in the Bellinger River in New South Wales. Routine veterinary health examinations of 17 healthy turtles were undertaken, along with the collection and analysis of blood samples, during conservation efforts to save the species following a catastrophic population decline. Microscopy analysis of blood films detected Haemoproteidae parasites that morphologically resembled Haemocystidium chelodinae inside turtle erythrocytes. Of the 17 turtles examined, 16 were positive for infection with H. chelodinae by both light microscopy and PCR. DNA sequencing of a partial fragment of the mitochondrial cytochrome b (cytb) gene and phylogenetic analysis identified two different H. chelodinae-like genotypes. The phylogenetic relationship of H. chelodinae-like to other Haemoproteidae species based on cytb sequences grouped H. chelodinae-like into the reptile clade, but revealed the Haemocystidium genus to be paraphyletic as the clade also contained Haemoproteus, thus supporting a re-naming of Haemoproteus species from reptiles to Haemocystidium species. This study reports for the first time the genetic characterisation of H. chelodinae-like organisms isolated from a new Testudine host species, the Bellinger River snapping turtle. As evidence grows, further research will be necessary to understand the mode of transmission and to investigate whether these parasites are pathogenic to their hosts.



Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Bensch S, Stjernman M, Hasselquist D, Ostman O, Hansson B, Westerdahl H, Pinheiro RT (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc Biol Sci 267(1452):1583–1589
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL (2005) GenBank. Nucleic Acids Res 33(Database Issue):D34–D38
Castellani A, Willey A (1904) Observations of the haematozoa of vertebrates in Ceylon. Spolia Zeylan 2:78–92
DeGiusti DL, Sterling CR, Dobrzechowski D (1973) Transmission of the chelonian haemoproteid Haemoproteus metchnikovi by a Tabanid fly Chrysops callidus. Nature 242(5392):50–51
Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5(1)
Fallon SM, Ricklefs RE (2008) Parasitemia in PCR-detected Plasmodium and Haemoproteus infections in birds. J Avian Biol 39(5):514–522
Garnham PCC (1966) Malaria parasites and other haemosporidia. Blackwell, Oxford
Hellgren O, Waldenstrom J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90(4):797–802
Hewitt RI (1940) Haemoproteus metchnikovi (Simond, 1901) from the yellow-bellied terrapin (Pseudemys elegans). J Parasitol 26:273–278
Jakes KA, O'Donoghue P, Munro M, Adlard R (2001) Hemoprotozoa of freshwater turtles in Queensland. J Wildl Dis 37(1):12–19
Jakes K, O'Donoghue PJ, Cameron SL (2003) Phylogenetic relationships of Hepatozoon (Haemogregarina) boigae, Hepatozoon sp., Haemogregarina clelandi and Haemoproteus chelodina from Australian reptiles to other Apicomplexa based on cladistic analyses of ultrastructural and life-cycle characters. Parasitology 126(Pt 6):555–559
Johnston TH, Cleland JB (1909) On a new melanin-producing haematozoon from an Australian tortoise. J Proc R Soc NSW 43:97–103
Johnston TH, Cleland JB (1910) The haematozoa of Australian Reptilia. No. 1. Proc Linnean Soc NSW 35:677–685
Johnston TH, Cleland JB (1912) The haematozoa of Australian Reptilia. No. 2. J Proc R Soc NSW 36:479
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12):1647–1649
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Lainson R, Naiff RD (1998) Haemoproteus (Apicomplexa: Haemoproteidae) of tortoises and turtles. Proc Biol Sci 265(1400):941–949
Levine ND (1988) The protozoan phylum Apicomplexa, vol II. CRC Press, Boca Raton, Florida
Mackerras MJ (1961) The haematozoa of Australian reptiles. Australian Journal of Zoology 9:61–122
Maia JP, Harris DJ, Carranza S (2016) Reconstruction of the evolutionary history of Haemosporida (Apicomplexa) based on the cyt b gene with characterization of Haemocystidium in geckos (Squamata: Gekkota) from Oman. Parasitol Int 65(1):5–11
O'Donoghue P (2017) Haemoprotozoa: making biological sense of molecular phylogenies. IJP-PAW 6(3):241–256
Orkun O, Guven E (2013) A new species of Haemoproteus from a tortoise (Testudo graeca) in Turkey, with remarks on molecular phylogenetic and morphological analysis. J Parasitol 99(1):112–117
Parson W, Pegoraro K, Niederstatter H, Foger M, Steinlechner M (2000) Species identification by means of the cytochrome b gene. Int J Legal Med 114(1-2):23–28
Pineda-Catalan O, Perkins SL, Peirce MA, Engstrand R, Garcia-Davila C, Pinedo-Vasquez M, Alonso Aguirre A (2013) Revision of hemoproteid genera and description and redescription of two species of chelonian hemoproteid parasites. J Parasitol 99(6):1089–1098
Rozsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86(2):228–232
Schaer J, McMichael L, Gordon AN, Russell D, Matuschewski K, Perkins SL, Field H, Power M (2018) Phylogeny of Hepatocystis parasites of Australian flying foxes reveals distinct parasite clade. Int J Parasitol Parasites Wildl 7(2):207–212
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
Shortt HE (1922) Review of the position of the genus Haemocystidium (Castellani and Willey, 1904), with a description of two new species. Indian J Med Res 9:814–826
Telford SR (1996) Two new species of Haemocystidium Castellian & Willey (Apicomplexa : Plasmodiidae) from Pakistan lizards, and the support their meronts provide for the validity of the genus. Syst Parasitol 34:197–214
Waldenstrom J, Bensch S, Hasselquist D, Ostman O (2004) A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol 90(1):191–194
Wenyon CM (1926) Protozoology. William Wood and Company, New York
Zhang J, Finlaison DS, Frost MJ, Gestier S, Gu X, Hall J, Jenkins C, Parrish K, Read AJ, Srivastava M, Rose K, Kirkland PD (2018) Identification of a novel nidovirus as a potential cause of large scale mortalities in the endangered Bellinger River snapping turtle (Myuchelys georgesi). PLoS One 13(10)
Acknowledgements
We acknowledge staff of the NSW Office of Environment and Heritage, particularly Gerry McGilvray and Shane Ruming for their coordination of the Bellinger River snapping Turtle emergency response and conservation program. We also acknowledge Dr Karrie Rose for her veterinary expertise and advice and for conducting health examinations of the animals used in this study. The intellectual contribution of Professor Peter O’Donoghue is also acknowledged. We would like to thank Adam Skidmore for his continued work into monitoring and safeguarding the Bellinger River snapping turtle.
Funding
The NSW Office of Environment and Heritage, NSW Department of Primary Industries, NSW Local Lands Services, Western Sydney University and Taronga Conservation Society Australia provided considerable financial and logistical support to the conservation efforts to save the Bellinger River snapping turtle.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Animals were collected under NSW Office of Environment and Heritage Scientific License (SL101647), and General Licence MWL000102467 (Section 120 of the National Parks and Wildlife Act 1974).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Handling Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Austen, J.M., Hall, J., Zahedi, A. et al. Further characterisation of Haemocystidium chelodinae-like Haemoproteidae isolated from the Bellinger River snapping turtle (Myuchelys georgesi). Parasitol Res 119, 601–609 (2020). https://doi.org/10.1007/s00436-019-06547-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06547-9