Abstract
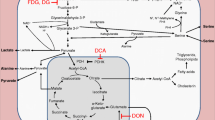
Besnoitia besnoiti is the causative agent of bovine besnoitiosis, a disease affecting both, animal welfare and cattle productivity. NETosis represents an important and early host innate effector mechanism of polymorphonuclear neutrophils (PMN) that also acts against B. besnoiti tachyzoites. So far, no data are available on metabolic requirements of B. besnoiti tachyzoite-triggered NETosis. Therefore, here we analyzed metabolic signatures of tachyzoite-exposed PMN and determined the relevance of distinct PMN-derived metabolic pathways via pharmacological inhibition experiments. Overall, tachyzoite exposure induced a significant increase in glucose and serine consumption as well as glutamate production in PMN. Moreover, tachyzoite-induced cell-free NETs were significantly diminished via PMN pre-treatments with oxamate and dichloroacetate which both induce an inhibition of lactate release as well as oxythiamine, which inhibits pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and transketolase, thereby indicating a key role of pyruvate- and lactate-mediated metabolic pathways for proper tachyzoite-mediated NETosis. Furthermore, NETosis was increased by enhanced pH conditions; however, inhibitors of MCT-lactate transporters (AR-C141900, AR-C151858) failed to influence NET formation. Moreover, a significant reduction of tachyzoite-induced NET formation was also achieved by treatments with oligomycin A (inhibitor of ATP synthase) and NF449 (purinergic receptor P2X1 antagonist) thereby suggesting a pivotal role of ATP availability for tachyzoite-mediated NETosis. In summary, the current data provide first evidence on carbohydrate-related metabolic pathways and energy supply to be involved in B. besnoiti tachyzoite-induced NETosis.









Similar content being viewed by others
References
Ahlin A, Gyllenhammar H, Ringertz BO, Palmblad J (1995) Neutrophil membrane potential changes and homotypic aggregation kinetics are pH-dependent: studies of chronic granulomatous disease. J Lab Clin Med 125:392–401
Amini P, Stojkov D, Felser A, Jackson CB, Courage C, Schaller A, Gelman L, Soriano ME, Nuoffer J-M, Scorrano L et al (2018) Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat Commun 9:2958
Aronsen L, Orvoll E, Lysaa R, Ravna AW, Sager G (2014) Modulation of high affinity ATP-dependent cyclic nucleotide transporters by specific and non-specific cyclic nucleotide phosphodiesterase inhibitors. Eur J Pharmacol 745:249–253
Baker VS, Imade GE, Molta NB, Tawde P, Pam SD, Obadofin MO, Sagay SA, Egah DZ, Iya D, Afolabi BB et al (2008) Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. Malar J 7:41
Bao Y, Ledderose C, Seier T, Graf AF, Brix B, Chong E, Junger WG (2014) Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J Biol Chem 289:26794–26803
Behnen M, Möller S, Brozek A, Klinger M, Laskay T (2017) Extracellular acidification inhibits the ROS-dependent formation of neutrophil extracellular traps. Front Immunol 8
Behrendt JH, Taubert A, Zahner H, Hermosilla C (2008) Studies on synchronous egress of coccidian parasites (Neospora caninum, Toxoplasma gondii, Eimeria bovis) from bovine endothelial host cells mediated by calcium ionophore A23187. Vet Res Commun 32:325–332
Behrendt JH, Ruiz A, Zahner H, Taubert A, Hermosilla C (2010) Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria bovis. Vet Immunol Immunopathol 133:1–8
Besnoit C, Robin V (1912) Sarcosporidiose cutanée chez une vache. Revue Vétérinaire 37:649–663
Bigalke, R. D., Prozesky, L., Coetzer, J. A. W. and Tustin, R. C. (2004). Infectious diseases of livestock.
Borregaard N, Herlin T (1982) Energy metabolism of human neutrophils during phagocytosis. J Clin Invest 70:550–557
Brinkmann V (2018) Neutrophil extracellular traps in the second decade. JIN:1–8
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535
Burnstock G (2007) Purine and pyrimidine receptors. Cell Mol Life Sci 64:1471–1483
Cao S, Liu P, Zhu H, Gong H, Yao J, Sun Y, Geng G, Wang T, Feng S, Han M et al (2015) Extracellular acidification acts as a key modulator of neutrophil apoptosis and functions. PLoS One 10:e0137221
Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314:1792–1795
Chen Y, Yao Y, Sumi Y, Li A, To, U. K., Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C et al (2010) Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal 3:ra45–ra45
Cortes H, Leitão A, Gottstein B, Hemphill A (2014) A review on bovine besnoitiosis: a disease with economic impact in herd health management, caused by Besnoitia besnoiti (Franco and Borges, ). Parasitology 141:1406–1417
Craven N, Williams MR, Field TR, Bunch KJ, Mayer SJ, Bourne FJ (1986) The influence of extracellular and phagolysosomal pH changes on the bactericidal activity of bovine neutrophils against Staphylococcus aureus. Vet Immunol Immunopathol 13:97–110
Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, García-Orozco A, Alvarado-Navarro A, Fafutis-Morris M (2017) Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol 8
Dhup S, Kumar Dadhich R, Ettore Porporato P, Sonveaux P (2012) Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des 18:1319–1330
DiStasi MR, Ley K (2009) Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol 30:547–556
European Food Safety Authority (2010) Bovine Besnoitiosis: an emerging disease in Europe. EFSA J 8
Fossati G, Moulding DA, Spiller DG, Moots RJ, White MRH, Edwards SW (2003) The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J Immunol 170:1964–1972
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176:231–241
Gabig TG, Bearman SI, Babior BM (1979) Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood 53:1133–1139
Gonzalez AS, Bardoel BW, Harbort CJ, Zychlinsky A (2014) Induction and quantification of neutrophil extracellular traps. Methods Mol Biol 1124:307–318
Grassi F (2010) Purinergic control of neutrophil activation. J Mol Cell Biol 2:176–177
Hahn, S., Giaglis, S., Chowdury, C. S., Hösli, I. and Hasler, P. (2013). Modulation of neutrophil NETosis: interplay between infectious agents and underlying host physiology. In Seminars in immunopathology, pp. 439–453. Springer.
Halestrap AP (2012) The monocarboxylate transporter family-structure and functional characterization. IUBMB Life 64:1–9
Halestrap AP (2013) The SLC16 gene family—structure, role and regulation in health and disease. Mol Asp Med 34:337–349
Jacobson KA, Ivanov AA, de Castro S, Harden TK, Ko H (2009) Development of selective agonists and antagonists of P2Y receptors. Purinergic Signal 5:75–89
Jacquiet P, Liénard E, Franc M (2010) Bovine besnoitiosis: epidemiological and clinical aspects. Vet Parasitol 174:30–36
Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E (2016) Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat Commun 7
Kellum, J. A., Song, M. and Li, J. (2004). Science review: extracellular acidosis and the immune response: clinical and physiologic implications. 8, 6.
Lacy P (2006) Mechanisms of degranulation in neutrophils. Allergy, Asthma Clin Immunol 2:98–108
Lardner A (2001) The effects of extracellular pH on immune function. J Leukoc Biol 69:522–530
Leblebicioglu B, Lim JS, Cario AC, Beck FM, Walters JD (1996) pH changes observed in the inflamed gingival crevice modulate human polymorphonuclear leukocyte activation in vitro. J Periodontol 67:472–477
Li S, Hao B, Lu Y, Yu P, Lee H-C, Yue J (2012) Intracellular alkalinization induces cytosolic Ca2+ increases by inhibiting sarco/endoplasmic reticulum Ca2 + -ATPase (SERCA). PLoS One 7
Maianski NA, Geissler J, Srinivasula SM, Alnemri ES, Roos D, Kuijpers TW (2004) Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death Differ 11:143
Maksimov P, Hermosilla C, Kleinertz S, Hirzmann J, Taubert A (2016) Besnoitia besnoiti infections activate primary bovine endothelial cells and promote PMN adhesion and NET formation under physiological flow condition. Parasitol Res 115:1991–2001
Malayev A, Nelson DJ (1995) Extracellular pH modulates the Ca 2+ current activated by depletion of intracellular Ca 2+ stores in human macrophages. J Membr Biol 146:101–111
Maqbool MS, Bhat SA, Shah SN, Ganayi BA, Sheikh TA (2012) Bovine Besnoitiosis-impact on profitable cattle production. International Journal of Livestock Research 2:78–81
Maueröder C, Mahajan A, Paulus S, Gößwein S, Hahn J, Kienhöfer D, Biermann MH, Tripal P, Friedrich RP, Munoz LE et al (2016) Ménage-à-Trois: the ratio of bicarbonate to CO2 and the pH regulate the capacity of neutrophils to form NETs. Front Immunol 7
Mazurek S, Michel A, Eigenbrodt E (1997) Effect of extracellular AMP on cell proliferation and metabolism of breast cancer cell lines with high and low glycolytic rates. J Biol Chem 272:4941–4952
Merezhinskaya N, Ogunwuyi SA, Mullick FG, Fishbein WN (2004) Presence and localization of three lactic acid transporters (MCT1, −2, and −4) in separated human granulocytes, lymphocytes, and monocytes. J Histochem Cytochem 52:1483–1493
Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A (2011) Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood 117:953–959
Muñoz-Caro T, Hermosilla C, Silva LMR, Cortes H, Taubert A, Kaltenboeck B (2014) Neutrophil extracellular traps as innate immune reaction against the emerging apicomplexan parasite Besnoitia besnoiti. PLoS ONE 9 (3):e91415. https://doi.org/10.1371/journal.pone.0091415.
Muñoz-Caro T, Conejeros I, Zhou E, Pikhovych A, Gärtner U, Hermosilla C, Kulke D, Taubert A (2018) Dirofilaria immitis Microfilariae and third-stage larvae induce canine NETosis resulting in different types of neutrophil extracellular traps. Front Immunol 9
Naffah de Souza C, Breda LCD, Khan MA, de Almeida SR, Câmara NOS, Sweezey N, Palaniyar N (2018) Alkaline pH Promotes NADPH oxidase-independent neutrophil extracellular trap formation: a matter of mitochondrial reactive oxygen species generation and citrullination and cleavage of histone. Front Immunol 8
Nahas GG, Tannieres ML, Lennon JF (1971) Direct measurement of leukocyte motility: effects of pH and temperature. Proc Soc Exp Biol Med 138:350–352
Nakagawa Y, Negishi Y, Shimizu M, Takahashi M, Ichikawa M, Takahashi H (2015) Effects of extracellular pH and hypoxia on the function and development of antigen-specific cytotoxic T lymphocytes. Immunol Lett 167:72–86
Nathan C (2006) Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6:173–182
Nayak MK, Dhanesha N, Doddapattar P, Rodriguez O, Sonkar VK, Dayal S, Chauhan AK (2018) Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood Advances 2:2029–2038
Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191:677–691
Parker H, Winterbourn C (2013) Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front Immunol 3
Pinheiro, C., Reis, R. M., Ricardo, S., Longatto-Filho, A., Schmitt, F. and Baltazar, F. (2010). Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. BioMed Research International.
Rabinovich M, DeStefano MJ, Dziezanowski MA (1980) Neutrophil migration under agarose: stimulation by lowered medium pH and osmolality. J Reticuloendothel Soc 27:189
Rada B, Jendrysik MA, Pang L, Hayes CP, Yoo D, Park JJ, Moskowitz SM, Malech HL, Leto TL (2013) Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PLoS One 8:e54205
Ratter JM, Rooijackers HMM, Hooiveld GJ, Hijmans AGM, de Galan BE, Tack CJ, Stienstra R (2018) In vitro and in vivo effects of lactate on metabolism and cytokine production of human primary PBMCs and monocytes. Front Immunol 9
Riemann A, Ihling A, Thomas J, Schneider B, Thews O, Gekle M (2015) Acidic environment activates inflammatory programs in fibroblasts via a cAMP–MAPK pathway. Biochim Biophys Acta (BBA) - Mol Cell Res 1853:299–307
Rodríguez-Espinosa O, Rojas-Espinosa O, Moreno-Altamirano MMB, López-Villegas EO, Sánchez-García FJ (2015) Metabolic requirements for neutrophil extracellular traps formation. Immunology 145:213–224
Ralevic V, Burnstock G (1998) Receptors for Purines and Pyrimidines. Pharmacological Reviews 50(3):413–92.
Seliger C, Leukel P, Moeckel S, Jachnik B, Lottaz C, Kreutz M, Brawanski A, Proescholdt M, Bogdahn U, Bosserhoff A-K et al (2013) Lactate-modulated induction of THBS-1 activates transforming growth factor (TGF)-beta2 and migration of glioma cells in vitro. PLoS One 8:e78935
Sil P, Hayes CP, Reaves BJ, Breen P, Quinn S, Sokolove J, Rada B (2017) P2Y6 Receptor antagonist MRS2578 inhibits neutrophil activation and aggregated neutrophil extracellular trap formation induced by gout-associated monosodium urate crystals. J Immunol 198:428–442
Silva LMR, Muñoz-Caro T, Burgos RA, Hidalgo MA, Taubert A, Hermosilla C (2016) Far beyond phagocytosis: phagocyte-derived extracellular traps act efficiently against protozoan parasites in vitro and in vivo. Mediat Inflamm
Simchowitz, L. (1985). Intracellular pH modulates the generation of superoxide radicals by human neutrophils.
Tanaka K, Koike Y, Shimura T, Okigami M, Ide S, Toiyama Y, Okugawa Y, Inoue Y, Araki T, Uchida K et al (2014) In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model. PLoS One 9:e111888
Taubert A, Hermosilla C, Silva LMR, Wieck A, Failing K, Mazurek S (2016) Metabolic signatures of Besnoitia besnoiti-infected endothelial host cells and blockage of key metabolic pathways indicate high glycolytic and glutaminolytic needs of the parasite. Parasitol Res 115:2023–2034
Trevani AS, Andonegui G, Giordano M, López DH, Gamberale R, Minucci F, Geffner JR (1999) Extracellular acidification induces human neutrophil activation. J Immunol 162:4849–4857
Tweedy, L., Knecht, D. A., Mackay, G. M. and Insall, R. H. (2016). Self-generated chemoattractant gradients: attractant depletion extends the range and robustness of chemotaxis. PLoS Biol 14,.
Tylicki A, Czerniecki J, Dobrzyn P, Matanowska A, Olechno A, Strumilo S (2005) Modification of thiamine pyrophosphate dependent enzyme activity by oxythiamine in Saccharomyces cerevisiae cells. Can J Microbiol 51:833–839
Vaughan KR, Stokes L, Prince LR, Meis S, Kassack MU, Bingle CD, Sabroe I, Surprenant A, Whyte MKB (2007) Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol 179:8544–8553
Villagra-Blanco R, Silva LMR, Muñoz-Caro T, Yang Z, Li J, Gärtner U, Taubert A, Zhang X, Hermosilla C (2017) Bovine polymorphonuclear neutrophils cast neutrophil extracellular traps against the abortive parasite Neospora caninum. Front Immunol 8
Villagra-Blanco R, Silva LMR, Conejeros I, Taubert A, Hermosilla C (2019) Pinniped- and Cetacean-derived ETosis contributes to combating emerging apicomplexan parasites (Toxoplasma gondii, Neospora caninum) circulating in marine environments. Biology 8:12
Wang X, Chen D (2018) Purinergic regulation of neutrophil function. Front Immunol 9
Wang J, Zhang X, Ma D, Lee W-NP, Xiao J, Zhao Y, Go VL, Wang Q, Yen Y, Recker R et al (2013) Inhibition of transketolase by oxythiamine altered dynamics of protein signals in pancreatic cancer cells. Exp Hematol Oncol 2:18
Wang X, Qin W, Xu X, Xiong Y, Zhang Y, Zhang H, Sun B (2017) Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation. Proc Natl Acad Sci U S A 114:4483–4488
Wu C-Y, Satapati S, Gui W, Wynn RM, Sharma G, Lou M, Qi X, Burgess SC, Malloy C, Khemtong C et al (2018) A novel inhibitor of pyruvate dehydrogenase kinase stimulates myocardial carbohydrate oxidation in diet-induced obesity. J Biol Chem 293:9604–9613
Zhou E, Conejeros I, Velásquez ZD, Muñoz-Caro T, Gärtner U, Hermosilla C, Taubert A (2019) Simultaneous and positively correlated NET formation and autophagy in Besnoitia besnoiti tachyzoite-exposed bovine polymorphonuclear neutrophils. Front Immunol 10:1131
Zigmond SH, Hargrove RL (1981) Orientation of PMN in a pH gradient: acid-induced release of a chemotactic factor. J Immunol 126:478–481
Acknowledgments
The authors would like to acknowledge Anika Seipp (Institute of Anatomy and Cell Biology, Justus Liebig University Giessen, Germany) for her excellent assistance in processing samples for scanning electron microscopy analysis. Many thanks to Prof. Mazurek’s assistants Sandra Rühl and Bianca Kulik for their help in measuring metabolites and creating the metabolic scheme. We also want to thank to Hannah Salecker and Dr. Christin Ritter for the technical support in B. besnoiti cell culture. We further thank all staff members of JLU Gießen teaching and research station Oberer Hardthof.
Funding
This research was funded by the German Research Foundation (Deutsche Forschungsgemeinsachaft, DFG, grant no. TA291/4-2). EZ is a recipient of a PhD fellowship from China Scholarship Council (file number: 201506170042).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Daniel K. Howe
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 785 kb)
Rights and permissions
About this article
Cite this article
Zhou, E., Conejeros, I., Gärtner, U. et al. Metabolic requirements of Besnoitia besnoiti tachyzoite-triggered NETosis. Parasitol Res 119, 545–557 (2020). https://doi.org/10.1007/s00436-019-06543-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06543-z