Abstract
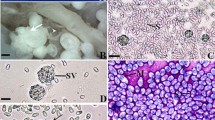
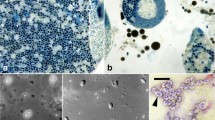
Infection with a new microsporidium, Fibrillaspora daphniae g. n. sp. n., was found in a local Daphnia magna population in Tomsk region (Western Siberia, Russia) at the prevalence rate of 52%. Histological sections showed parasite cells entirely encompassing the host haemocoel. Methanol-fixed spores were elongate, oval, 4.8 ± 0.3 μm × 2.3 ± 0.2 μm in size. All developmental stages were in direct contact with the host cell cytoplasm, with single nuclei, and division by binary fission. The sporont surface was covered with an additional outer layer composed of fine tubules. The spores possessed a thick endospore, large posterior vacuole filled with electron-dense granules, and a bipartite polaroplast composed of anterior lamellar and posterior globular elements. The polar tube was slightly anisofilar, with 13–19 coils arranged in one row; the two posterior coils were of lesser diameter. The small subunit ribosomal RNA gene sequence was deposited in Genbank under accession # MF278272. Considering the sister relationship between Fibrillanosema crangonycis and our new isolate described here as Fibrillaspora daphniae, we propose a new family Fibrillasporidae fam. n. to contain these two genera and the descendants of their common ancestor. A new superfamily Tubulinosematoidea superfam. n. is proposed as a monophyletic assemblage of Fibrillasporidae fam. n. and Tubulinosematidae.





Similar content being viewed by others
Change history
23 February 2018
Ultrastructure of Fibrillaspora is highly similar to that of Berwaldia but the former is phylogenetically distant from the taxon annotated as Berwaldia schaefernai (Genbank accession # AY090042). It was shown recently, however, that the latter sequence was not derived from Berwaldia, and correct nucleotide sequence data were provided for Berwaldia. This allowed recondideration of Fibrillaspora as a junior synonym of Berwaldia and redefinition of Fibrillaspora daphniae as a new combination, Berwaldia daphniae comb. nov.
References
Choudhary MM, Metcalfe MG, Arrambide K, Bern C, Visvesvara GS, Pieniazek NJ, Bandea RD, Deleon-Carnes M, Adem P, Choudhary MM, Zaki SR, Saeed MU (2011) Tubulinosema sp. microsporidian myositis in immunosuppressed patient. Emerg Infect Dis 17(9):1727–1730. https://doi.org/10.3201/eid1709.101926
Ebert D (1995) The ecological interactions between a microsporidian parasite and its host Daphnia magna. J Animal Ecol 64(3):361–369. https://doi.org/10.2307/5897
Ebert D (2005) Ecology, epidemiology, and evolution of parasitism in Daphnia. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Available from: http://www.ncbi. nlm.nih.gov/books/NBK2036
Franzen C, Fischer S, Schroeder J, Schölmerich J, Schneuwly S (2005) Morphological and molecular investigations of Tubulinosema ratisbonensis gen. nov., sp. nov. (Microsporidia: Tubulinosematidae fam. nov.), a parasite infecting a laboratory colony of Drosophila melanogaster (Diptera: Drosophilidae). J Eukaryot Microbiol 52(2):141–152. https://doi.org/10.1111/j.1550-7408.2005.04-3324.x
Franzen C, Futerman PH, Schroeder J, Salzberger B, Kraaijeveld AR (2006) An ultrastructural and molecular study of Tubulinosema kingi Kramer (Microsporidia: Tubulinosematidae) from Drosophila melanogaster (Diptera: Drosophilidae) and its parasitoid Asobara tabida (Hymenoptera: Braconidae). J Invertebr Pathol 91(3):158–167. https://doi.org/10.1016/j.jip.2005.11.007
Green J (1974) Parasites and epibionts of Cladocera. Trans Zool Soc London 32:417–515
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp 41:95–98
Haag KL, James TY, Pombert JF, Larsson R, Schaer TM, Refardt D, Ebert D (2014) Evolution of a morphological novelty occurred before genome compaction in a lineage of extreme parasites. Proc Natl Acad Sci U S A 111(43):15480–15485. https://doi.org/10.1073/pnas.1410442111
Issi IV (1986) Microsporidia as a phylum of parasitic protozoa. In: Beyer TV, Issi IV (eds) Protozoology 10. Leningrad. Nauka, pp 1–136
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evolut 16(2):111–120. https://doi.org/10.1007/BF01731581
Kumar S, Stecher G, Tamura K (2015) MEGA7: molecular evolutionary genetics analysis version 7.0. Mol Biol Evol 33(7):1870–1874
Larsson JIR, Ebert D, Vávra J, Voronin VN (1996) Redescription of Pleistophora intestinalis Chatton, 1907, a microsporidian parasite of Daphnia magna and Daphnia pulex, with establishment of the new genus Glugoides (Microspora, Glugeidae). Europ J Protistol 32(2):251–261. https://doi.org/10.1016/S0932-4739(96)80024-1
Larsson RJI (2005) Fixation of microsporidian spores for electron microscopy. J Invertebr Pathol 90(1):47–50. https://doi.org/10.1016/j.jip.2005.06.016
Lukyantsev VV, Simakova AV (2014) Infestation of lower crustaceans (Copepoda, Cladocera) with microsporidians (Microsporidia) in Western Siberia. Parazitologiya 48(5):358–372
Manuilova EF (1964) Cladocerans of fauna of the USSR. M.; L: Nauka. 318 p
Meissner EG, Bennett JE, Qvarnstrom Y, da Silva A, Chu EY, Tsokos M, Gea-Banacloche J (2012) Disseminated microsporidiosis in an immunosuppressed patient. Emerg Infect Dis 18(7):1155–1158. https://doi.org/10.3201/eid1807.120047
Refardt D, Canning EU, Marthis A, Cheney SA, Lafranchi-Tristem NJ, Ebert D (2002) Small subunit ribosomal DNA phylogeny of microsporidia that infect Daphnia (Crustacea: Cladocera). Parasitology 124(Pt 4):381–389
Refardt D, Decaestecker В, Jonson PTJ, Vavra J (2008) Morphology, molecular phylogeny, and ecology of Binucleata daphniae n. g., n. sp. (Fungi: Microsporidia), a parasite of Daphnia magna Straus, 1820 (Crustacea: Branchiopoda). Eukaryot Microbiol 55(5):393–408. https://doi.org/10.1111/j.1550-7408.2008.00341.x
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17(1):208–212. https://doi.org/10.1083/jcb.17.1.208
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Slothouber Galbreath JGM, Smith JE, Terry RS, Becnel JJ, Dunn AM (2004) Invasion success of Fibrillanosema crangonycis n.sp., n,g.: a novel vertically transmitted microsporidian parasite from the invasive amphipod host Crangonyx pseudogracilis. Internat. J Parasitol 34:235–244
Sprague V, Becnel JJ, Hazard EI (1992) Taxonomy of phylum Microspora. Crit Rev Microbiol 18(5-6):285–395. https://doi.org/10.3109/10408419209113519
Tokarev Y, Sitnikova N, Pistone D, Lizhi Luo, Huang Shaozhe, Ignatieva A, Senderskiy I, Toderas I, Frolov A (2010) Microsporidia PCR detection artifacts due to non-specific binding of the universal microsporidia primers to the rDNA of arthropod hosts. Buletinul ASM Stiintele Vietii 2010. 310, 72–77
Tokarev Y, Voronin V, Rusakovich E, Issi I (2014) Detection of microsporidia in Gammarids in the Delta of the Kuban River (Azov Sea, Russia). 47th annual meeting of the Society of Invertebrate Pathology. Julius Kuhn Institute, p 86
Undeen AH, Vavra J (1997) Research methods for entomopathogenic Protozoa. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, pp 117–151. https://doi.org/10.1016/B978-012432555-5/50010-5
Vavra J, Larsson JIR (1994) Berwaldia schaefernai (Jirovec, 1937) comb. n. (Protozoa, Microsporidia), fine structure, life cycle and relationship to Bervaldia singularis Larsson, 1981. Europ J Protistol 30(1):45–54. https://doi.org/10.1016/S0932-4739(11)80197-5
Vavra J, Hylis M, Fiala J, Nebesarova J (2016) Globulispora mitoportans n.g., n.sp. (Opisthosporidia: Microsporidia) a microsporidian parasite of daphnids with spore organization and prominent mitosome-like vesicles. J Invertebr Pathol 135:43–52. https://doi.org/10.1016/j.jip.2016.02.003
Vidtmann SS, Sokolova YY (1994) The description of the new genus Larssonia gen. n. based on the ultrastructural analysis of Microsporidium (Pleistophora) obtusa from Daphnia pulex (Cladocera). Parazitologyia 28:202–213
Voronin VN, Issi IV (1974) Methods of working with microsporidians. Parazitologiya 8(3):272–273
Vossbrinck CR, Debrunner-Vossbrinck BA (2005) Molecular phylogeny of the microsporidia: ecological, ultrastructural and taxonomic considerations. Folia Parasitol 52(1):131–142. https://doi.org/10.14411/fp.2005.017
Vossbrinck CR, Debrunner-Vossbrinck BA, Weiss LM (2014) Molecular phylogeny of the Microsporidia. In: Weiss LM, Becnel JJ (eds.). Microsporidia: Pathogens of Opportunity. Wiley-Blackwell 203–220
Weigl S, Korner H, Petrusek A, Seda J, Wolinska J (2012) Natural distribution and co-infection patterns of microsporidia parasites in the Daphnia longispina complex. Parasitology 139(07):870–880. https://doi.org/10.1017/S0031182012000303
Weiser J (1977) Contribution of the classification of Microsporidia. Vestn Cs spolec zool 41(4):308–320
Weiss LH, Vossbrinck CR (1999) Molecular biology, molecular phylogeny, and molecular diagnostic approaches to the microsporidia. In: The Microsporidia and Microsporidiosis (Eds. M. Wittner, L. M. Weiss). ASM Press, Washington, 129–171, DOI: https://doi.org/10.1128/9781555818227.ch4
Acknowledgements
Authors are indebted to A.A. Miller (Institute of Strength Physics and Materials Science of Siberian Branch Russian Academy of Sciences, Tomsk, Russia) for assistance with transmission electron microscopy and to Theodore G. Andreadis (The Connecticut Agricultural Experiment Station, CT, USA) for language correction of the manuscript. The research is performed within the frames of Governmental Contract from Ministry of Education and Science of Russian Federation # 6.7525.2017/8.9 and a grant from Russian Foundation of Basic Research # 17-04-00871.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simakova, A.V., Tokarev, Y.S. & Issi, I.V. A new microsporidium Fibrillaspora daphniae g. n. sp. n. infecting Daphnia magna (Crustacea: Cladocera) in Siberia and its taxonomic placing within a new family Fibrillasporidae and new superfamily Tubulinosematoidea (Opisthosporidia: Microsporidia). Parasitol Res 117, 759–766 (2018). https://doi.org/10.1007/s00436-018-5749-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5749-2