Abstract
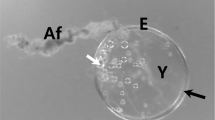
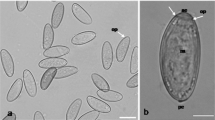
Eggs obtained from the uteri of female nematodes, genetically identified as Contracaecum multipapillatum s.l., found in a brown pelican (Pelecanus occidentalis) from Bahía de La Paz, Gulf of California, Mexico, were used to study the early developmental stages of this anisakid by scanning electron microscopy (SEM). Egg dimensions were approximately 54 × 45 μm measured by SEM. Observation of the eggs revealed an outer surface of fibrous appearance. The newly hatched larvae were ensheathed and highly motile. Observation with SEM showed that the sheaths of the larvae were striated and revealed an excretory pore and a cleft near the anterior end of the sheath, presumably to facilitate the opening of the sheath for the emergence of the larva. The hatched larvae were placed in nutritive culture medium, where they grew within their sheath, some exsheathing completely 2 weeks later. The surface patterns of the sheath and the cuticle of the exsheathed larvae were clearly different. Although they did not moult during culture, SEM revealed a morphology typical of third-stage larvae of Contracaecum from fish, as previously observed by optical microscopy. Thus, we suggest that newly hatched larvae from eggs of C. multipapillatum are third larval stage but with sheath of the second larval stage, as occuring in other anisakids.







Similar content being viewed by others

References
Adroher FJ, Malagón D, Valero A, Benítez R (2004) In vitro development of the fish parasite Hysterothylacium aduncum from the third larval stage recovered from a host to the third larval stage hatched from the egg. Dis Aquat Org 58:41–45. doi:10.3354/dao058041
Anderson RC (2000) Nematode parasites of vertebrates: their development and transmission, 2nd edn. CAB International, Wallingford
Chandler AC (1935) Parasites of fishes in Galveston Bay. Proc United States Natl Museum 83:123–157. doi:10.5479/si.00963801.83-2977.123
Cruthers CR, Weise RW, Hanson MF (1974) Topography of Ascaridia galli eggs exposed to low temperatures or cryoprotectants as shown by scanning electron microscopy. J Parasitol 60:632–635. doi:10.2307/3278726
Davey JT (1969) The early development of Contracaecum osculatum. J Helminthol 43:293–298. doi:10.1017/S0022149X00004843
Deardorff TL, Overstreet RM (1980) Contracaecum multipapillatum (=C. robustum) from fishes and birds in the northern Gulf of Mexico. J Parasitol 66:853–856. doi:10.2307/3280688
Dziekońska-Rynko J, Rokicki J, Wziatek B (2008) Experimental infection of Carassius auratus (L., 1758) with the second stage larvae of the nematode Contracaecum rudolphii Hartwich, 1964. Wiad Parazytol 54:339–343
Fernández Bargiela J (1987) Los parásitos de la lisa Mugil cephalus L., en Chile: sistemática y aspectos poblacionales (Perciformes: Mugilidae). Gayana Zool 51:3–58
González L (1998) The life cycle of Hysterothylacium aduncum (Nematoda: Anisakidae) in Chilean marine farms. Aquaculture 162:173–186. doi:10.1016/S0044-8486(97)00303-7
Huizinga HW (1966) Studies on the life cycle and development of Contracaecum spiculigerum (Rudolphi, 1809) (Ascaroidea: Heterocheilidae) from marine piscivorous birds. J Elisha Mitchell Sci Soc 82:181–195
Huizinga HW (1967) The life cycle of Contracaecum multipapillatum (von Drasche, 1882) Lucker, 1941 (Nematoda: Heterochelidae). J Parasitol 53:368–375. doi:10.2307/3276593
Humphrey S, Courtney C, Forrester DJ (1978) Community ecology of the helminth parasites of the brown pelican. Wilson Bull 90:587–598. doi:10.2307/4161129
Iglesias L, Gómez del Prado MC, Adroher FJ, Valero A (1998) Floridosentis elongatus y Contracaecum sp., parásitos de Mugil cephalus en Baja California Sur, México. In: First Virtual Congress about Pharmacy (IVCP). Granada, Spain, p C068
Iglesias L, Benítez R, Adroher FJ, Valero A (2011) Helminth infection in Mugil incilis from Cartagena de Indias, Colombian Caribbean coast. Helminthologia 48:36–40. doi:10.2478/s11687-011-0007-5
Kimura M (1980) A simple method for estimating evolutionary rate of base substitution through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Køie M (1993) Aspects of the life cycle and morphology of Hysterothylacium aduncum (Rudolphi, 1802) (Nematoda, Ascaridoidea, Anisakidae). Can J Zool 71:1289–1296. doi:10.1139/z93-178
Køie M, Fagerholm HP (1993) Third-stage larvae emerge from eggs of Contracaecum osculatum (Nematoda, Anisakidae). J Parasitol 79:777–780. doi:10.2307/3283621
Køie M, Berland B, Burt MDB (1995) Development to third-stage larvae occurs in the eggs of Anisakis simplex and Pseudoterranova decipiens (Nematoda, Ascaridoidea, Anisakidae). Can J Fish Aquat Sci 52:134–139. doi:10.1139/f95-519
Lucker JT (1941) A redescription of Contracaecum multipapillatum (Von Drasche, 1882) (Nematoda: Anisakinae). J Parasitol 27:505–512. doi:10.2307/3272525
Malagón D, Benítez R, Kašný M, Adroher FJ (2013) Peptidases in parasitic nematodes: a review. In: Erzinger GS (ed) Parasites: ecology, diseases and management. Nova Science Publishers, Hauppage, pp 61–102
Mattiucci S, Paoletti M, Solorzano AC, Nascetti G (2010) Contracaecum gibsoni n. sp. and C. overstreeti n. sp. (Nematoda: Anisakidae) from the Dalmatian pelican Pelecanus crispus (L.) in Greek waters: genetic and morphological evidence. Syst Parasitol 75:207–224. doi:10.1007/s11230-009-9220-8
Measures LN, Hong H (1995) The number of moults in the egg of sealworm, Pseudoterranova decipiens (Nematoda: Ascaridoidea): an ultrastructural study. Can J Fish Aquat Sci 52:156–160. doi:10.1139/f95-521
Moravec F (2009) Experimental studies on the development of Contracaecum rudolphii (Nematoda: Anisakidae) in copepod and fish paratenic hosts. Folia Parasitol (Praha) 56:185–193. doi:10.14411/fp.2009.023
Rello Yubero FJ, Adroher Auroux FJ, Valero López A (2004) Anisákidos parásitos de peces comerciales. Riesgos asociados a la salud pública. An Real Acad Ciencias Vet Andalucía Orient 17:173–198
Smith JW, Elarifi AE, Wootten R, Pike AW, Burt MDB (1990) Experimental infection of rainbow trout, Oncorhynchus mykiss, with Contracaecum osculatum (Rudolphi, 1802) and Pseudoterranova decipiens (Krabbe, 1878) (Nematoda: Ascaridoidea). Can J Fish Aquat Sci 47:2293–2296. doi:10.1139/f90-255
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Thomas LJ (1937) On the life cycle of Contracaecum spiculigerum (Rud.) J Parasitol 23:429–431. doi:10.2307/3272243
Ubelaker JE, Allison VF (1975) Scanning electron microscopy of the eggs of Ascaris lumbricoides, A. suum, Toxocara canis, and T. mystax. J Parasitol 61:802–807. doi:10.2307/3279211
Valles-Ríos ME, Ruiz-Campos G, Galavíz-Silva L (2000) Prevalencia e intensidad parasitaria en Mugil cephalus (Pisces: Mugilidae), del Rio Colorado, Baja California, Mexico. Rev Biol Trop 48:495–501
Valles-Vega GI (2011) Supervivencia de larvas III de Contracaecum multipapillatum sensu lato (Nematoda: Anisakidae) de lisas (Pisces: Mugilidae) en diferentes medios culinarios y aspectos sobre su ciclo de vida en La Paz, B.C.S. Tesis de Grado. Universidad Autónoma de Baja California Sur, La Paz, Mexico
Valles-Vega I, Gómez del Prado-Rosas MC (2014) Changes in the anatomical organization of Contracaecum multipapillatum L3 sensu lato (Nematoda: Anisakidae) larvae subjected to different culinary treatments in La Paz, Baja California Sur, Mexico. Comp Parasitol 81:165–174. doi:10.1654/4609.1
Valles-Vega I, Molina-Fernández D, Benítez R, Hernández-Trujillo S, Adroher FJ (2017) Early development and life cycle of Contracaecum multipapillatum s.l. from a brown pelican Pelecanus occidentalis in Gulf of California, Mexico. Dis Aquat Organ 125:167–178. doi:10.3354/dao03147
Valter ED, Popova TI, Valovaya MA (1982) Scanning electron microscope study of four species of anisakid larvae (Nematoda: Anisakidae). Helminthologia 19:195–209
Vidal-Martínez VM, Osorio-Sarabia D, Overstreet RM (1994) Experimental infection of Contracaecum multipapillatum (Nematoda: Anisakinae) from Mexico in the domestic cat. J Parasitol 80:576–579. doi:10.2307/3283194
Weerasooriya MV, Fujino T, Ishii Y, Kagei N (1986) The value of external morphology in the identification of larval anisakid nematodes: a scanning electron microscope study. Z Parasitenkd 72:765–778. doi:10.1007/BF00925097
Wharton D (1980) Nematode egg-shells. Parasitology 81:447–463. doi:10.1017/S003118200005616X
Yagi K, Nagasawa K, Ishikura H, Nagasawa A, Sato N, Kikuchi K, Ishikura H (1996) Female worm Hysterothylacium aduncum excreted from human: a case report. Jpn J Parasitol 45:12–23
Zhu X-Q, D’Amelio S, Paggi L, Gasser RB (2000) Assessing sequence variation in the internal transcribed spacers of ribosomal DNA within and among members of the Contracaecum osculatum complex (Nematoda: Ascaridoidea: Anisakidae). Parasitol Res 86:677–683. doi:10.1007/PL00008551
Acknowledgements
The authors are grateful to Dr. Bárbara González Acosta (from Microbiology lab of CICIMAR, La Paz, Mexico) for her help with the genetic identification of the parasites. Translation to English was by Robert Abrahams, BSc.
Funding information
This work has been funded by the Ministerio de Economía y Competitividad (Spain) and European Regional Development Fund (ERDF) (DMF, FJA and RB; grant number CGL2013-47725-P); and by the Instituto Politécnico Nacional, Mexico (IVV and SHT; grant number SIP20141443).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Molina-Fernández, D., Valles-Vega, I., Hernández-Trujillo, S. et al. A scanning electron microscopy study of early development in vitro of Contracaecum multipapillatum s.l. (Nematoda: Anisakidae) from a brown pelican (Pelecanus occidentalis) from the Gulf of California, Mexico. Parasitol Res 116, 2733–2740 (2017). https://doi.org/10.1007/s00436-017-5583-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5583-y