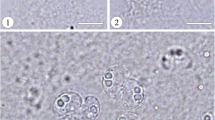
Abstract
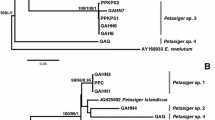
Zygocercous (aggregating) cercarial larvae were recently discovered emerging from a physid snail during a molecular survey of cercariae from molluscs in lakes in central Alberta, Canada. This manuscript delves into the characterization of these cercariae through morphological and molecular techniques and provides the first genetic information for a zygocercous larval trematode. Analyses of cytochrome c oxidase I of mitochondrial DNA and two partial regions of nuclear ribosomal DNA sequences revealed the zygocercous cercariae to belong to the genus Australapatemon Sudarikov, 1959. Further analyses of sequences of Australapatemon burti (Miller, 1923), from cercariae and adults collected from across North America, indicate a complex of nine genetically-distinct lineages within this species, a surprising level of diversity. The zygocercous cercariae, along with adult worms collected from ducks in Manitoba, Canada, and from Mexico, represent one of these lineages, and are herein described as Australapatemon mclaughlini n. sp. Seven lineages cannot yet be identified, but one is tentatively identified as Australapatemon burti.



Similar content being viewed by others
References
Beuret J, Pearson JC (1994) Description of a new zygocercous cercaria (Opisthorchioidea: Heterophyidae) from prosobranch gastropods collected at Heron Island (Great Barrier Reef, Australia) and a review of zygocercariae. Syst Parasitol 27:105–125. doi:10.1007/BF00012269
Blair D (1976) Observations on the life-cycle of the strigeoid trematode, Apatemon (Apatemon) gracilis (Rudolphi, 1819) Szidat, 1928. J Helminthol 50:125–131. doi:10.1017/S0022149X00027607
Blasco-Costa I, Locke SA (2017) Life history, systematics and evolution of the Diplostomoidea Poirier, 1886: progress, promises and challenges emerging from molecular studies. Adv Parasitol. doi:10.1016/bs.apar.2017.05.001
Blasco-Costa I, Poulin R, Presswell B (2016) Species of Apatemon Szidat, 1928 and Australapatemon Sudarikov, 1959 (Trematoda: Strigeidae) from New Zealand: linking and characterising life cycle stages with morphology and molecules. Parasitol res 115:271–289. doi:10.1007/s00436-015-4744-0
Cable RM (1956) Marine cercariae of Puerto Rico. In: scientific survey of Porto Rico and Virgin Islands. Vol XVI-Part 4. pp 491–577
Cable RM (1963) Marine cercariae from curacao and Jamaica. Zeitschrift für Parasitenkd 23:429–469
Cable RM, McLean RA (1943) The occurrence of Cercaria clausii Monticelli, a marine Rattenkönig larval trematode, on the west coast of Florida. Not Naturae 129:1–7
Caffara M, Locke SA, Gustinelli A et al (2011) Morphological and molecular differentiation of Clinostomum complanatum and Clinostomum marginatum (Digenea: Clinostomidae) metacercariae and adults. J Parasitol 97:884–891. doi:10.1645/GE-2781.1
Committee ABR (2015) The Official List of Birds of Alberta. http://www.royalalbertamuseum.ca/research/lifeSciences/ornithology/birdlist/taxonomy.cfm
Cort WW, Brooks ST (1928) Studies on the Holostome cercariae from Douglas Lake, Michigan. Trans am Microsc Soc 47:179. doi:10.2307/3222175
Davies D, Ostrowski de Núñez M (2012) The life cycle of Australapatemon magnacetabulum (Digenea: Strigeidae) from northwestern Argentina. J Parasitol 98:778–783
Drago FB, Lunaschi LI (2010) Digenea, Strigeidae, Australapatemon canadensis Dubois and Rausch, 1950: first record in South America and a new host record. Check List 6:382. doi:10.15560/6.3.382
Drago F, Lunaschi L, Hinojosa-Saez A, González-Acuña D (2007) First record of Australapatemon burti and Paramonostomum pseudalveatum (Digenea) from Anas georgica (Aves, Anseriformes) in Chile. Acta Parasitol 52:201–205. doi:10.2478/s11686-007-0040-1
Dronen NO Jr (1973) Studies on macrocercous cercariae of the Douglas Lake, Michigan area. Trans am Microsc Soc 92:641–648
Dubois G (1968) Synopsis des Strigeidae et des Diplostomatidae (Trematoda). Mém Soc Sci Nat Neuchatel 10:1–258
Dubois G (1988) Quelques Strigeoidea (Trematoda) récoltés au Paraguay par les expéditions du Muséum d’Histoire naturelle de Genève, au cours des années 1979, 1982 et 1985. Rev Suisse Zool 95:521–532. doi:10.5962/bhl.part.79670
Ebbs ET, Loker ES, Davis NE et al (2016) Schistosomes with wings: how host phylogeny and ecology shape the global distribution of Trichobilharzia querquedulae (Schistosomatidae). Int J Parasitol 46:669–677. doi:10.1016/j.ijpara.2016.04.009
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids res 32:1792–1797. doi:10.1093/nar/gkh340
Faltýnková A, Niewiadomska K, Santos MJ, Valtonen ET (2007) Furcocercous cercariae (Trematoda) from freshwater snails in Central Finland. Acta Parasitol 52:310–317. doi:10.2478/s11686-007-0050-z
Galazzo DE, Dayanandan S, Marcogliese DJ, McLaughlin JD (2002) Molecular systematics of some North American species of Diplostomum (Digenea) based on rDNA-sequence data and comparisons with European congeners. Can J Zool 80:2207–2217. doi:10.1139/z02-198
García-Varela M, Sereno-Uribe AL, Pinacho-Pinacho CD et al (2016) Molecular and morphological characterization of Austrodiplostomum ostrowskiae Dronen, 2009 (Digenea: Diplostomatidae), a parasite of cormorants in the Americas. J Helminthol 90:174–185. doi:10.1017/S0022149X1500005X
Gordy MA, Kish L, Tarrabain M, Hanington PC (2016) A comprehensive survey of larval digenean trematodes and their snail hosts in central Alberta, Canada. Parasitol Res 115:3867–3880. doi:10.1007/s00436-016-5152-9
Guindon S, Dufayard JF, Lefort V et al (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi:10.1093/sysbio/syq010
Harley CM, Cienfuegos J, Wagenaar DA (2011) Developmentally regulated multisensory integration for prey localization in the medicinal leech. J Exp Biol 214:3801–3807
Harley CM, Rossi M, Cienfuegos J, Wagenaar D (2013) Discontinuous locomotion and prey sensing in the leech. J Exp Biol 216:1890–1897
Hendrickson GL, Kingston N (1974) Cercaria laramiensis sp. n., a freshwater zygocercous cercaria from Physa gyrina say, with a discussion of cercarial aggregation. J Parasitol 60:777–781
Hernández-Mena DI, García-Prieto L, García-Varela M (2014) Morphological and molecular differentiation of Parastrigea (Trematoda: Strigeidae) from Mexico, with the description of a new species. Parasitol Int 63:315–323. doi:10.1016/j.parint.2013.11.012
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogeny. Bioinformatics 17:754–755. doi:10.1093/bioinformatics/17.8.754
Johnston TH, Angel LM (1951) The morphology and life cycle of the trematode Apatemon intermedius from the black swan. Trans R Soc South Aust 74:66–78
Kearse M, Moir R, Wilson A et al (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi:10.1093/bioinformatics/bts199
Komiya Y (1941) A new “zygocercaria”, Cercaria radiata, and its excretory system (cercaria from Chinese fresh waters no. 3). J Shanghai Sci Inst [ns] 1:229–232
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:msw054. doi:10.1093/molbev/msw054
de León Pérez-Ponce G, Poulin R (2017) An updated look at the uneven distribution of cryptic diversity among parasitic helminths. J Helminthol:1–6. doi:10.1017/S0022149X17000189
de León Pérez-Ponce G, Garcia-Varela M, Pinacho-Pinacho CD et al (2016) Species delimitation in trematodes using DNA sequences: middle-American Clinostomum as a case study. Parasitology 143:1773–1789. doi:10.1017/S0031182016001517
Lepage D, Vaidya G, Guralnick R (2014) Avibase–a database system for managing and organizing taxonomic concepts. Zookeys 420:117–135. doi:10.3897/zookeys.420.7089
Littlewood DTJ, Olson PD (2001) Small subunit rDNA and the Platyhelminthes: signal, noise, conflict and compromise. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor & Francis, London, pp 262–278
Locke SA, Al-Nasiri FS, Caffara M et al (2015a) Diversity, specificity and speciation in larval Diplostomidae (Platyhelminthes: Digenea) in the eyes of freshwater fish, as revealed by DNA barcodes. Int J Parasitol 45:841–855. doi:10.1016/j.ijpara.2015.07.001
Locke SA, Caffara M, Marcogliese DJ, Fioravanti ML (2015b) A large-scale molecular survey of Clinostomum (Digenea, Clinostomidae). Zool Scr 44:203–217. doi:10.1111/zsc.12096
López Z, Cárdenas L, Runil F, González MT (2015) Contrasting definitive hosts as determinants of the genetic structure in a parasite with complex life cycle along the south-eastern Pacific. Mol Ecol 24:1060–1073. doi:10.1111/mec.13080
Martin WE (1968) Cercaria gorgoncephala Ward, 1916, a zygocercous species in northwestern United States. Trans am Microsc Soc 87:472–476
Martin WE, Gregory VL (1951) Cercaria buchanani n.sp., an aggregating marine trematode. Trans am Microsc Soc 70:359–362
McCarthy AM (1990) Experimental observations on the specificity of Apatemon (Australapatemon) minor (Yamaguti 1933) (Digenea: Strigeidae) toward leech (Hirudinea) second intermediate hosts. J Helminthol 64:161–167. doi:10.1017/S0022149X00012074
McDonald ME (1981) Key to trematodes reported in waterfowl. United States Department of the Interior. Fish and Wildlife Service, Fort Collins, p 156
Miller HM (1923) Notes on some furcocercous larval trematodes. J Parasitol 10:35–46
Miller HM (1926) Comparative studies on furcocercous cercariae. University of Illinois biological monographs 10:1–112
Miller HM (1929) Continuation of study on behavior and reactions of marine cercariae from Tortugas. Carnegie Institute of Washington Yearbook No.28 (for 1928–29) pp 292–294
Miller HM (1930) Formations and behaviour of aggregations of cercariae. J Parasitol 17:111–112
Miura O, Kuris A, Torchin M (2005) Molecular-genetic analyses reveal cryptic species of trematodes in the intertidal gastropod, Batillaria cumingi (Crosse). Int J Parasitol 35:793–801
Moszczynska A, Locke SA, McLaughlin JD et al (2009) Development of primers for the mitochondrial cytochrome c oxidase I gene in digenetic trematodes (Platyhelminthes) illustrates the challenge of barcoding parasitic helminths. Mol Ecol Resour 9(Suppl 1):75–82. doi:10.1111/j.1755-0998.2009.02634.x
Negm-Eldin M, Davies RW (2002) Morphology and life cycle of Apatemon hypseleotris species novum from Australia including metacercariae viability and excystment. Dtsch Tierarztl Wochenschr 109:314
Niewiadomska K (2002) Family Strigeidae Railliet, 1919. In: Gibson DI, Jones A, Bray RA (eds) Keys to the Trematoda. Wallingford: CABI publishing and the Natural History Museum, London, pp 231–241
Oliva ME, Valdivia IM, Chavez RA et al (2015) Molecular and morphological evidence demonstrating two species of Helicometrina Linton 1910 (Digenea: Opecoelidae) in northern Chile. J Parasitol 101:694–700. doi:10.1645/14-523
Pintner T (1891) Ueber Cercaria clausii Monticelli. Arb Aus Dem Zool Insituten der Univ Wein 9:285–294
Pleijel F, Jondelius U, Norlinder E et al (2008) Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Mol Phylogenet Evol 48:369–371. doi:10.1016/j.ympev.2008.03.024
Poulin R, de León Pérez-Ponce G (2016) Data from: global analysis reveals that cryptic diversity is linked with habitat but not mode of life. J Evol Biol 30(3):1420–9101. doi:10.5061/DRYAD.1HJ46
Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, automatic barcode gap discovery for primary species delimitation. Mol Ecol 21:1864–1877. doi:10.1111/j.1365-294X.2011.05239.x
Stunkard HW, Willey CH, Rabinowitz Y (1941) Cercaria burti Miller, 1923, a larval stage of Apatemon gracilis (Rudolphi, 1819) Szidat, 1928. Trans am Microsc Soc 60:485–497. doi:10.2307/3222768
Tatonova YV, Chelomina GN, Besprosvannykh VV (2012) Genetic diversity of nuclear ITS1-5.8S-ITS2 rDNA sequence in Clonorchis sinensis Cobbold, 1875 (Trematoda: Opisthorchidae) from the Russian far east. Parasitol Int 61:664–674. doi:10.1016/j.parint.2012.07.005
Ulmer MJ (1970) Notes on rearing of snails in the laboratory. In: Experiments and techniques in parasitology. W. H. Freeman and Co. San Francisco, pp 143–144
Van Steenkiste N, Locke SA, Castelin M et al (2014) New primers for DNA barcoding of digeneans and cestodes (Platyhelminthes). Mol Ecol Resour 15:945–952. doi:10.1111/1755-0998.12358
Vilas R, Criscione CD, Blouin MS (2005) A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology 131:839–846. doi:10.1017/S0031182005008437
Vojtek J (1964) Zur Kenntnis des Entwicklungszyklus von Apatemon cobitidis (Linstow, 1890). Zeitschrift für Parasitenkd 24:578–599. doi:10.1007/BF00328726
Ward HB (1916) Notes on two free-living larval trematodes from North America. J Parasitol 3:10–20
Wardle WJ (1988) A Bucephalid larva, Cercaria pleuromerae n.Sp. (Trematoda:Digenea), parasitizing a deepwater bivalve from the Gulf of Mexico. J Parasitol 74:692–694
Acknowledgements
We thank Arlene Oatway of the Advanced Microscopy Facility at the University of Alberta for her assistance with SEM processing and imaging. We also thank Lisa Kish, Valerie K. Phillips, and Mahmoud Tarrabain for assistance with field collections and sample processing in Alberta, where work was funded by Alberta Innovates Energy and Environment Solutions grant 2078 and 2332, and National Sciences and Engineering Research Council (NSERC) grant 418540 (PCH), and in Manitoba by NSERC grant A6979 to J. Daniel Mclaughlin. MAG was partly supported by an NSERC CREATE Host-Parasite Interactions student scholarship. SAL was supported by the Puerto Rico Science, Technology and Research Trust. We also thank Pieter Johnson (University of Colorado), for providing samples from California, and David J. Marcogliese (Environment and Climate Change Canada) for encouraging early phases of this study, which was also funded by the Canadian Federal Government’s Genomics Research Development Initiative, NSERC Discovery Grant (A6979), and by Paul D. N. Hebert at the Center for Biodiversity Genomics, University of Guelph, Canada through funding from NSERC, Genome Canada, the Ontario Genomics Institute and the International Barcode of Life initiative. We acknowledge two anonymous reviewers for their constructive comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Suppl. Fig. 1
Bayesian inference phylograms of the Strigeidae and outgroups (Diplostomidae) derived from a) partial 28S, and b) partial ITS1–5.8S rDNA gene sequences with posterior probability values followed by bootstrap proportions given above branches. Values less than 0.5 are not reported. Scale bars denote number of substitutions per site. (PDF 3077 kb).
Suppl. Table 1
Review of zygocercous and aggregating cercariae. (XLSX 14 kb).
Suppl. Table 2
Estimates of evolutionary divergence over sequence pairs between and within groups among three genetic markers (cox1, 28S, ITS1–5.8S–ITS2). Each marker is represented by a matrix. Below the diagonal is the number of base pair differences per site from averaging over all sequence pairs between groups (interspecific divergence values), otherwise referred to as p distance values. Values pertaining specifically to the genus Australapatemon are within the grey box. Minimum and maximum mean interspecific divergence values among A. burti lineages are in bold and underlined. Standard error estimates are based on 1000 bootstrap replicates and above the diagonal and are coloured blue. The number of base pair differences per site from averaging over all sequence pairs within each group (intraspecific divergence values) is shown along the diagonal in red. Missing values, represented by a minus sign, are present for groups that contain singletons, and therefore estimates cannot be made. For cox1, the analysis involved 120 nucleotide sequences with a total of 399 positions in the final dataset. For 28S, the analysis involved 14 nucleotide sequences with a total of 807 positions in the final dataset. Finally, for ITS1–5.8S–ITS2, the analysis involved 26 nucleotide sequences with a total of 482 positions in the final dataset (partial ITS1–5.8 s after final trimming). All positions containing gaps and missing data were eliminated. (XLSX 15 kb).
Suppl. Table 3
Statistics for morphometric comparisons of cercariae of lineages 1, 6, 7, 8, and 9 with those of Australapatemon (Cercaria) burti (Miller, 1923), Cercaria laramiensis (Hendrickson & Kingston, 1974), and Cercaria absurda (Miller, 1927). Statistical tests used were Kruskal-Wallis analysis of variance (H), and post hoc multiple comparisons test, with Bonferroni correction for multiple tests. Statistical significance (α = 0.05) is indicated by bold text and an asterisk. Only significant comparisons between lineages/species are listed. (PDF 27 kb).
Suppl. Table 4
Statistics for significant comparisons between cercaria within LIN1. Statistics reported are from a post hoc multiple comparisons test, with Bonferroni correction for multiple tests. Statistical significance (α = 0.05) is indicated by bold text and an asterisk. (PDF 11 kb).
Suppl. Table 5
Summary data from samples from which DNA sequences were obtained. (XLSX 15.9 kb).
Suppl. Table 6
Comparison of morphology of adults in four genetically distinguished lineages of Australapatemon and A. mclaughlini n. sp., with ten species of Australapatemon. Listed are differences between the lineage and the species (e.g., adults of A. mclaughlini n. sp. are smaller than A. anseris, but have larger eggs). TL=total length; OS=oral sucker; PH=pharynx; VS=ventral sucker; FB=forebody; HB=hindbody; HB:FB=hindbody length/forebody length; AT=anterior testis; PT=posterior testis; GC=genital cone. (XLSX 11 kb).
Rights and permissions
About this article
Cite this article
Gordy, M.A., Locke, S.A., Rawlings, T.A. et al. Molecular and morphological evidence for nine species in North American Australapatemon (Sudarikov, 1959): a phylogeny expansion with description of the zygocercous Australapatemon mclaughlini n. sp.. Parasitol Res 116, 2181–2198 (2017). https://doi.org/10.1007/s00436-017-5523-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5523-x