Abstract
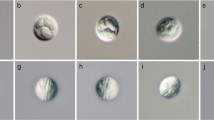
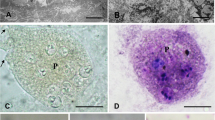
The traditional taxonomy of the genus Chloromyxum Mingazzini, 1890 has been intensively challenged to be paraphyletic by recent ribosomal DNA (rDNA)-based phylogenetic analysis. Undersampling to get rich sequence data to infer more scientific phylogenetic relationships makes scientists conservatively assign all non-marine elasmobranch-infecting species as Chloromyxum sensu lato. Although complex ridge pattern on the spore surface observed by scanning electron microscopy was thought to be critical for the identification of Chloromyxum species, insufficient data also prevent this ultrastructural data to be a valid taxonomic feature for this genus. It is especial for Chloromyxum species to be reported in China. Molecular and ultrastructural characteristics are yet available for all 22 Chloromyxum species recorded in China. During the investigation of the diversity of coelozoic fish myxosporeans, Chloromyxum ellipticum Li & Nie, 1973 was found to highly infect the gall bladder of Ctenopharyngodon idellus Valenciennes, 1844 in Poyang Lake watershed of Jiangxi province, Eastern China. Here, we redescribed it by the currently recommended holistic approach of combining morphological, ultrastructural, and molecular characteristics. Mature spores were found floating free in the gall bladder, but no plasmodium observed. Spores are typical freshwater teleost-infecting Chloromyxum species, spherical or subspherical in lateral view, measuring 7.7 ± 0.08 μm (6.9–9.1) in length, 6.3 ± 0.09 μm (5.6–7.6) in width, and 5.8 ± 0.20 μm (5.2–6.3) in thickness. Four pyriform polar capsules, located at the anterior end of the spores, were equal in size, 3.3 ± 0.06 μm (2.2–4.1) long and 2.1 ± 0.03 μm (1.7–2.5) wide. Polar filaments coiled with four to five turns. Two equal spore valves are symmetrical, with 10–16 surface extrasutural ridges per valve, aligned along the longitudinal axis. The obtained partial 18S rDNA of C. ellipticum did not match any sequences available in GenBank. Phylogenetic analysis showed that C. ellipticum clustered firstly with Chloromyxum legeri with robust nodal support and grouped then with urinary system of freshwater teleost-infecting Chloromyxum clade, rather than other gall bladder of freshwater teleost-infecting clade.




Similar content being viewed by others
References
Carriero MM, Adriano EA, Silva MRM, Ceccarelli PS, Maia AAM (2013) Molecular phylogeny of the Myxobolus and Henneguya genera with several new South American species. PLoS One 8:1–12
Chen QL, Ma CL (1998) Fauna Sinica, Myxozoa: Myxosporea. Science Press, Beijing In Chinese
Eiras JC, Lu YS, Gibson DI, Fiala I, Saraiva A, Cruz C, Santos MJ (2012) Synopsis of the species of Chloromyxum Mingazzini, 1890 (Myxozoa: Myxosporea: Chloromyxidae). Syst Parasitol 83:203–225
Fiala I (2006) The phylogeny of Myxosporea (Myxozoa) based on small subunit ribosomal RNA gene analysis. Int J Parasitol 36:1521–1534
Fiala I, Bartošová P (2010) History of myxozoan character evolution on the basis of rDNA and EF-2 data. BMC Evol Biol 10:228
Fiala I, Dyková I (2004) The phylogeny of marine and freshwater species of the genus Chloromyxum Mingazzini, 1890 (Myxosporea: Bivalvulida) based on small subunit ribosomal RNA gene sequences. Parasitol Res 51:211–214
Fiala I, Bartošová P, Whipps CM (2015) Classification and phylogenetics of myxozoa In: Myxozoan evolution, ecology and development by Okamura B, Gruhl A & Bartholomew JL. Springer, ISBN978–3–319-14753-6 (eBook), pp: 85–110
Gleeson RJ, Adlard RD (2012) Phylogenetic relationships amongst Chloromyxum Mingazzini, 1890 (Myxozoa: Myxosporean), and the description of six novel species from Australian elasmobranchs. Parasitol Int 61:267–274
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hallett SL, Atkinson SD, Holt RA, Banner CR, Bartholomew JL (2006) A new myxozoan form feral goldfish (Carassius auratus). J Parasitol 92:357–363
Holzer AS, Sommerville C, Wooten R (2006) Molecular identity, phylogeny and life cycle of Chloromyxum schurovi Shul’man & leshko 2003. Parasitol Res 99:90–96
Jirků M, Fiala I, Modrý D (2007) Tracing the genus Sphaerospora: rediscovery, redescription and phylogeny of the Sphaerospora ranae (Morelle, 1929) n. comb. (Myxosporea, Sphaerosporidae), with emendation of the genus Sphaerospora. Parasitology 134:1727–1739
Jirků M, Bartošová P, Kodádková A, Mutschmann F (2011) Another Chloromyxid lineage: molecular phylogeny and redescription of Chloromyxum careni from the Asian horned frog Megophrys nasuta. J Eukaryot Microbio 58(1):50–59
Lom J, Arthur JR (1989) A guideline for preparation of species descriptions in Myxosporea. J Fish Dis 12:151–156
Lom J, Dyková I (1993) Scanning electron microscopic revision of common species of the genus Chloromyxum (Myxozoa: Myxosporea) infecting European freshwater fishes. Folia Parasitol 40:161–174
Lom J, Dyková I (2006) Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol 53:1–36
Page RDM (1996) TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Rocha S, Graca C, Al-Quraishy S, Azevedo C (2013) Morphological and molecular characterization of a new myxozoan species (Myxosporea) infecting the gall bladder of Raja clavata (Chondrichthyes), from the Portuguese Atlantic coast. J Parasitol 99(2):307–317
Rocha S, Graca C, Al-Quraishy S, Azevedo C (2014) Morphological and ultrastructural redescription of Chloromyxum leydigi Mingazzini, 1890 (Myxozoa: Myxosporea), type species of the genus, infecting the gall bladder of the marine cartilaginous fish Torpedo marmorata Risso (Chondrichthyes: Torpedinidae), from the Portuguese Atlantic coast. Folia Parasitol 61(1):1–10
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Sanders JL, Jaramillo AG, Ashford JE, Feist SW, Lafferty KD, Kent ML (2015) Two myxozoans from the urinary tract of topsmelt, Atherinops affinis. J Parasitol 101(5):577–586
Shin SP, Nguyen VG, Jeong JM, Jun JW, Kim JH, Han JE, Baeck GW, Park SC (2014) The phylogenetic study on Thelohanellus species (Myxosporea) in relation to host specificity and infection site tropism. Mol Phylogenet Evol 72:31–34
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Whipps CM, Adlard RD, Bryant MS, Lester RJG, Findlay V, Kent ML (2003) First report of three Kudoa species from eastern Australia: Kudoa thyrsites from mahi mahi (Coryphaena hippurus), Kudoa amamiensis and Kudoa minithyrsites n. sp. from sweeper (Pempheris ypsilychnus). J Eukuryot Microbio 50:215–219
Acknowledgements
We appreciated much the help of Mr. DW Zhang of Administration Bureau of Liaofang Reservoir, Jiangxi Province, for collecting the fish specimens. The present work was financially supported by the Natural Sciences Foundation of China (31472296), Jiangsu Fishery Project (D2015-11), Basic project SB Russian Academy of Science (VI.51.1.3), and Russian Fund of Basic Research (14-04-91176 GFEN_a).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, J.Y., Zhao, Y.L., Batueva, M. et al. Redescription of Chloromyxum ellipticum Li & Nie, 1973 (Myxosporea: Chloromyxidae) infecting the gall bladder of grass carp Ctenopharyngodon idellus Valenciennes, 1844, supplemented by morphological and molecular characteristics. Parasitol Res 116, 1479–1486 (2017). https://doi.org/10.1007/s00436-017-5424-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5424-z