Abstract
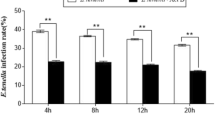
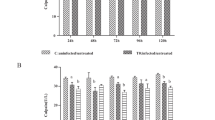
Accumulating evidence suggests that Eimeria tenella severely damages the intestinal mucosa in infected poultry, resulting in deadly haemorrhagic typhlocolitis and major economic losses. Damage to host tissue is believed to arise mainly from apoptosis, which is, in general, intimately related to mitochondrial function. However, it is unclear whether mitochondria-dependent apoptotic pathways are specifically involved in parasite-induced apoptosis of chick embryo cecal epithelial cells. Because the mitochondrial permeability transition pore (MPTP) and caspase-9 are important elements in these pathways, we studied the effects of their respective inhibitors (i.e., cyclosporine A [CsA] and Z-LEHD-FMK, respectively) in primary cultures of chicken embryonic cecum epithelial cells using histopathological techniques, terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assays, flow cytometry (FCM) and ELISA. Results indicated that the inhibitors significantly decreased (p < 0.01) DNA injury, apoptosis and caspase-9 and caspase-3 activity of chick embryo cecal epithelial cells at 24, 48, 72, 96 and 120 h after E. tenella infection. Thus, our data supported that mitochondria-dependent apoptotic pathways were involved in apoptosis of parasitised chick embryo cecal epithelial cells.







Similar content being viewed by others
References
Allen PC, Fetterer RH (2002) Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev 1(15):58–65
Antonsson B, Montessuit S, Sanchez B, Martinou JC (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem 276(15):11615–11623
Bachaya HA, Raza MA, Khan MN, Iqbal Z, Abbas RZ, Murtaza S, Badar N (2012) Predominance and detection of different Eimeria species causing coccidiosis in layer chickens. J Anim Plant Sci 22(3):597–600
Cacho E, Gallego MF, Lopez BF, Quı´lez J, Sa´nchez AC (2004) Expression of anti-apoptotic factors in cells parasitized by second-generation schizonts of Eimeria tenella and Eimeria necatrix. Vet Parasitol 125:287–300
Chawla-Sarkar M, Leaman DW, Borden EC (2001) Preferentia1 induction of apoptosis by interferon(IFN)-β compared with IFN-α2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res 7(6):l821–l1831
Cho SG, Choi FJ (2002) Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol 35(1):24–27
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341:233–249
Cui XZ, Zheng MX, Zhang Y, Liu RL, Yang SS, Li S, Xu ZY, Bai R, Lv QH, Zhao WL (2016) Calcium homeostasis in mitochondrion-mediated apoptosis of chick embryo cecal epithelial cells induced by Eimeria tenella infection. Res Vet Sci 104:166–173
Donepudi M, Sweeney AM, Briand C, Grütter MG (2003) Insights into the regulatory mechanism for caspase-8 activation. Mol Cell 11(2):543–549
Du J, Di HS, Guo L, Li ZH, Wang GL (2008) Hyperthermia causes bovine mammary epithelial cell death by a mitochondrial-induced pathway. J Therm Biol 33(1):37–47
Frias MA, Rebsamen MC, Gerber WC, Lang U (2007) Prostaglandin E2 activation Stat3 in neonatal rat ventricular cardiomyocytes: a role in cardiac hypertrophy. Cardiovasc Res 73:57–65
Green DR (2000) Apoptotic pathways: paper wraps stone blunts scissors. Cell 102:1–4
Gu SP, Zheng MX, Li BJ, Han KG (2010) Analysis on apoptosis of chicken cecal epithelial cells infected by E. tenella. Acta Vet Zootechnica Sin 41(10):1322–1327
Gurevich RM, Regula KM, Kirshenbaum LA (2001) Serpin protein CrmA suppresses hypoxia-mediated apoptosis of ventricular myocytes. Circ J 103(15):1984–1991
Hady MM, Zaki MM (2012) Efficacy of some herbal feed additives on performance and control of cecal coccidiosis in broilers. APCBEE Procedia 4:163–168
Haritova AM, Lashev LD, Koinarski VC (2013) Sulfachloropyrazine disposition in Eimeria tenella infected chickens. Veterinarski Arhiv 83(2):211–222
Hirpara JL, Seyed MA, Loh KW, Dorg H, Kini RM, Pervaiz S (2000) Induction of mitochondrial permeability transition and cytochrome C release in the absence of caspase activation is insufficient for effective apoptosis in human leukemia cells. Blood 95(9):1773–1780
Kass GEN (2006) Mitochondrial involvement in drug-induced hepatic injury. Chem Biol Interact 163(1–2):145–159
Kaufmann SH, Hengartner MO (2001) Programmed cell death: alive and well in the new millennium. Trends Cell Biol 11(12):526–534
Kinnally KW, Peixoto PM, Ryu SY, Dejean LM (2011) Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta 1813(4):616–622
Kipper M, Andretta I, Lehnen CR, Lovatto PA, Monteiro SG (2013) Meta-analysis of the performance variation in broilers experimentally challenged by Eimeria spp. Vet Parasitol 196(1–2):77–84
Kong M, Ba MW, Liang H, Tian L (2012) Cyclosporin A attenuates effect of β-amyloid peptide25-35 on apoptosis of PCI2 cells by inhibiting endoplasmic reticulum stress. Chin J Geriatr Heart Brain Vessel Dis 14(7):760–762
Lang M, Kann M, Zahner H, Taubert A, Hermosilla C (2009) Inhibition of host cell apoptosis by Eimeria bovis sporozoites. Vet Parasitol 160(1–2):25–33
Leirião P, Rodrigues CD, Albuquerque SS, Mota MM (2004) Survival of protozoan intracellular parasites in host cells. EMBO Rep 5:1142–1147
Leshnower BG, Kanemoto S, Matsubara M, Sakamoto H, Hinmon R, Gorman RC (2008) Cyclosporine preserves mitochondrial morphology after myocardial ischemia/reperfusion independent of calcineurin inhibition. Ann Thorac Surg 86(4):1286–1292
Martynova-Van Kley MA, Oviedo-Rondon EO, Dowd SE, Hume M, Nalian A (2012) Effect of Eimeria infection on cecal microbiome of broilers fed essential oils. Int J Poult Sci 11(12):747–755
Mo PH, Ma QT, Zhao X, Cheng N, Tao JP, Li J (2014) Apoptotic effects of antimalarial artemisinin on the second generation merozoites of Eimeria tenella and parasitized host cells. Vet Parasitol 206(3–4):297–303
Peng YT, Chen P, Ouyang RY, Song L (2015) Multifaceted role of prohibitin in cell survival and apoptosis. Apoptosis 20(9):1135–1149
Pop C, Timmer J, Sperandio S, Salvesen GS (2006) The apoptosome activates caspase-9 by dimerization. Mol Cell 22(2):269–275
Sheng ZG, Cao XJ, Peng SQ, Wang CY, Li QQ, Wang YM, Liu MF (2008) Ofloxacin induces apoptosis in microencapsulated juvenile rabbit chondrocytes by caspase-8-dependent mitochondrial pathway. Toxicol Appl Pharmacol 226(2):119–127
Strasser A, Jost PJ, Nagata S (2009) The many roles of FAS receptor signaling in the immune system. Immunity 30(2):180–192
Takuhashi M, Kigawa J, Minagawa Y, Itamochi H, Shimada M, Kamazawa S, Sato S, Akeshima R, Terakawa N (2000) Sensitivity to paclitaxel is not related to p53-dependent apoptosis in ovarian cancer cells. Eur J Cancer 36(14):1863–1868
Walter DH, Haendeler J, Galle J, Zeiher AM, Dimmeler SD (1998) Cyclosporin a inhibits apoptosis of human endothelial cells by preventing release of cytochrome C from mitochondria. Circulation 98(12):1153–1157
Wang Z, Shen JZ, Suo X, Zhao SY, Cao XY (2006) Experimentally induced monensin-resistant Eimeria tenella and membrane fluidity of sporozoites. Vet Parasitol 138(3–4):186–193
Wang HW, Zhou BH, Cao JL, Gu XL, Cao CF, Wang JD (2009a) Effects of dietary protein and calcium on thymus apoptosis induced by fluoride in female rats (wistar rats). Environ Toxicol 24(3):218–224
Wang L, Luo HS, Xia H (2009b) Role of caspase activation in butyrate induced apoptosis of HT-29 colon carcinoma cells. Chin J Pathophysiol 25(10):1950–1953
Xu ZY, Zheng MX, Zhang Y, Cui XZ, Yang SS, Li S, Lv QH, Zhao WL, Bai R (2016) The effect of the mitochondrial permeability transition pore on apoptosis in Eimeria tenella host cells. Poult Sci. doi:10.3382/ps/pew198
Yadavilli S, Martinez CE, Snowden AJ, Hurst A, Joseph T, Albrecht T, Muganda PM (2007) Diepoxybutane activates the mitochondrial apoptotic pathway and mediates apoptosis in human lymphoblasts through oxidative stress. Toxicol In Vitro 21(8):1429–1441
Yang SS, Zheng MX, Xu HC, Cui XZ, Zhang Y, Zhao WL, Bai R (2015) The effect of mitochondrial ATP-sensitive potassium channels on apoptosis of chick embryo cecal cells by Eimeria tenella. Res Vet Sci 99:188–195
Zhang Y, Zheng MX, Xu ZY, Xu HC, Cui XZ, Yang SS, Li S, Lv QH, Bai R (2015) Relationship between Eimeria tenella development and host cell apoptosis in chickens. Poult Sci 94(12):2970–2979
Zheng MX, Gu SP, Wang LX, Han KG, Li BJ (2011) Effect on E. tenella invasion into host cells in vitro by inhibiting calcium signal transduction. Sci Agric Sin 44(15):3272–3278
Zhou B, Wang H, Xue F, Wang X, Fei C, Wang M, Zhang T, Yao X, He P (2010) Effects of diclazuril on apoptosis and mitochondrial transmembrane potential in second-generation merozoites of Eimeria tenella. Vet Parasitol 168(3–4):217–220
Acknowledgments
This study was funded by a grant from the National Natural Science Foundation of China (Grant No. 31272536).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All work involving animals was conducted in accordance with the national legislations concerning the protection of animal welfare and following the strict guidelines and approval of the Institution Animal Care and Use Committee of Shanxi Agricultural University, China.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00436-017-5390-5.
Rights and permissions
About this article
Cite this article
Li, S., Zheng, Mx., Xu, Hc. et al. Mitochondrial pathways are involved in Eimeria tenella-induced apoptosis of chick embryo cecal epithelial cells. Parasitol Res 116, 225–235 (2017). https://doi.org/10.1007/s00436-016-5283-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5283-z