Abstract
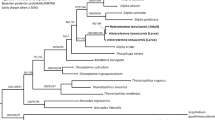
During the last decade, many morphotypes of larval forms of Hysterothylacium have been proposed; however, their boundaries are taxonomically unclear. We compare newly collected material with data available for Hysterothylacium larvae, based on genetics and morphology, in order to evaluate their relationships and simplify their classification. The previously described Hysterothylacium larval type IV, type V, and type X were found in several marine fishes from Brazil. A new Hysterothylacium larval type XV, parasitic in Elops saurus from State of Sergipe, north-eastern Brazil, was proposed based on its genetic singularity and the presence of numerous spines throughout the body. Based upon phylogenetic reconstruction using the ribosomal DNA (rDNA) ITS2 sequences along with morphological traits, the following third stage larvae were considered valid (synonyms are inside parenthesis) type III (= types VII and VIII), type V (= types VI, XII, and XIII), and type X (= type XIV). Despite the lack of molecular data, Hysterothylacium larval type II is considered as synonym of type I because of their identical morphology. The morphometry of Hysterothylacium third stage larvae proved to be weak as a diagnostic tool due to its high rates of variation. Phylogenetic reconstruction using the concatenated sequences of rDNA ITS1–5.8S–ITS2 and the mitochondrion DNA (mtDNA) cox2 revealed that the sampled Hysterothylacium larval types V may represent three different species and H. deardorffovestreetorum may represent two different taxa. Furthermore, H. deardorffovestreetorum is considered species inquirenda due to its problematic description and diagnosis which are based only on larvae. A key to Hysterothylacium larvae from marine fishes is provided.




Similar content being viewed by others
References
Anderson RC (2000) Nematode parasites of vertebrates: their development and transmission. CAB International, Wallingford, 672
Anderson RC, Chabaud AG, Willmott S (2009) Keys to the nematode parasites of vertebrates, Archival Volume. CAB International, Wallingford, 463
Arizono N, Yoshimura Y, Tohzaka N, Yamada M, Tegoshi T, Onishi K, Uchikawa R (2010) Ascaris in Japan: is pig-derived Ascaris infecting humans? Jpn J Infect Dis 63:447–448
Billings AN, Yu X, Teel PD, Walker DH (1998) Detection of a spotted fever group rickettsia in Amblyoma cajennense (Acari: Ixodidae) in South Texas. J Medical Entomol 35:474–478. doi:10.1093/jmedent/35.4.474
Borges JN, Cunha LFG, Santos HLC, Monteiro-Neto C, Santos CP (2012) Morphological and molecular diagnosis of anisakid nematode larvae from cutlassfish (Trichiurus lepturus) off the coast of Rio de Janeiro, Brazil. PLoS One 7:e40447. doi:10.1371/journal.pone.0040447
Cannon LRG (1977) Some larval ascaridoids from South-eastern Queensland marine fishes. Int J Parasitol 7:233–243. doi:10.1016/0020-7519(77)90053-4
Darriba D, Taboada GL, Ramón D, Posada D (2012) jModelTest 2: more models, new heuristics and high-performance computing. Nat Methods 9:772. doi:10.1038/nmeth.2109
Fonseca MCG, Knoff M, Felizardo NN, Di Azevedo MIN, Torres EJL, Gomes DC, Iñiguez AM, São Clemente SC (2016) Integrative taxonomy of Anisakidae and Raphidascarididae (Nematoda) in Paralichthys patagonicus and Xystreurys rasile (Pisces: Teleostei) from Brazil. Int J Food Microbiol. doi:10.1016/j.ijfoodmicro.2016.07.026
Froese R, Pauly D (2015) FishBase. World Wide Web electronic publication. www.fishbase.org, version (10/2015)
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. doi:10.1080/10635150390235520
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. doi:10.1093/bioinformatics/17.8.754
Jabbar A, Asnoussi A, Norbury LJ, Eisenbarth A, Shamsi S, Gasser RB, Lopata AL, Beveridge I (2012) Larval anisakid nematodes in teleost fishes from Lizard Island, northern Great Barrier Reef, Australia. Mar Freshwater Res 63:1283–1299. doi:10.1071/MF12211
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast fourier transform. Nucleic Acids Res 30:3059–3066. doi:10.1093/nar/gkf436
Kijewska A, Rokicki J, Sitko J, Wegrzyn G (2002) Ascaridoidea: a simple DNA assay for identification of 11 species infecting marine and freshwater fish, mammals, and fish-eating birds. Exp Parasitol 101:35–39. doi:10.1016/S0014-4894(02)00031-0
Klimpel S, Palm HW (2001) Anisakid nematode (Ascaridoidea) life cycles and distribution. Increasing zoonotic potential in the time of climate change? Parasitol Res 2:201–222. doi:10.1007/978-3-642-21396-0_11
Knoff M, Felizardo NN, Iñiguez AM, Maldonado A Jr, Torres EJL, Pinto RM, Gomes DC (2012) Genetic and morphological characterization of a new species of the genus Hysterothylacium (Nematoda) from Paralichthys isoscles Jordan, 1890 (Pisces: Teleostei) of the Neotropical Region, state of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 107:186–198. doi:10.1590/S0074-02762012000200006
Luque JL, Aguiar JC, Vieira FM, Gibson DI, Santos CP (2011) Checklist of Nematoda associated with the fishes of Brazil. Zootaxa 3082:1–88. doi:10.11646/%25x
Mattiucci S, Cianchi R, Paggi L, Arduino P, Margolis L, Brattey J, Webb SC, D’Amelio S, Orecchia P, Bullini L (1997) Genetic and ecological data on the Anisakis simplex complex, with evidence for a new species (Nematoda, Ascaridoidea, Anisakidae). J Parasitol 83:401–416. doi:10.2307/3284394
Mattiucci S, Fazii P, De Rosa A, Paoletii M, Megna AS, Glielmo A, De Angelis M, Costa A, Mucci C, Calvaruso V, Sorrentini I, Palma G, Buschi F, Nascetti G (2013) Anisakiasis and gastroallergic reactions associated with Anisakis pegreffii infections, Italy. Emerg Infect Dis 19:496–499. doi:10.3201/eid1903.121017
Mattiucci S, Nascetti G, Dailey M, Webb SC, Barros NB, Cianchi R, Bullini L (2005) Evidence for a new species of Anisakis Dujardin, 1845: morphological description and genetic relationships between congeners (Nematoda: Anisakidae). Syst Parasitol 61:157–171. doi:10.1007/s11230-005-3158-2
Mattiucci S, Nascetti G (2008) Advances and trends in the molecular systematics of Anisakis nematodes, with implications for their evolutionary ecology and host-parasite co-evolutionary processes. Adv Parasitol 66:47–148. doi:10.1016/S0065-308X(08)00202-9
Mattiucci S, Paoletti M, Webb SC (2009) Anisakis nascetti s. sp. (Nematoda: Anisakidae) from beaked whales of the southern hemisphere: morphological description, genetic relationships between congeners and ecological data. Sys Parasitol 74:199–217. doi:10.1007/s11230-009-9212-8
Mattiucci S, Turchetto M, Brigantini F, Nascetti G (2002) On the occurrence of the sibling species of Contracaecum rudolphii complex (Nematoda: Anisakidae) in cormorants (Phalacrocorax carbo sinensis) from Venetian and Caorle lagoons: genetic markers and ecological studies. Parassitologia 44(Suppl1):105
Moravec F (1994) Parasitic nematode of freshwater fishes of Europe. Academia, Praha, 473
Moravec F (1998) Nematodes of freshwater fishes of the Neotropical Region. Academia, Praha, 464p
Moravec F, Justine J (2015) Anisakid nematodes (Nematoda: Anisakidae) from the marine fishes Plectropomus laevis Lacépède (Serranidae) and Sphyraena qenie Klunzinger (Sphyraenidae) off New Caledonia, including two new species of Hysterothylacium Ward & Magath, 1917. Syst Parasitol 92:181–195. doi:10.1007/s11230-015-9597-5
Moreira J, Paschoal F, Cezar AD, Luque JL (2015) Community ecology of the metazoan parasites of Brazilian sardinella, Sardinella brasiliensis (Steindachner, 1879) (Actinopterygii: Clupeidae) from the coastal zone of the State of Rio de Janeiro, Brazil. Braz J Biol 75:736–741. doi:10.1590/1519-6984.00114
Nadler SA, Hudspeth DSS (2000) Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: Hypotheses of structural and sequence evolution. J Parasitol 86:380–393. doi:10.2307/3284785
Nadler SA, D’Amelio S, Dailey MD, Paggi L, Siu S, Sakanari JA (2005) Molecular phylogenetics and diagnosis of Anisakis, Pseudoterranova and Contracaecum from Northern Pacific marine mammals. J Parasitol 91:1413–1429
Pantoja CS, Borges JN, Santos CP, Luque JL (2015) Molecular and morphological characterization of anisakid nematode larvae from sandperches Pseudopercis numida and Pinguipes brasilianus (Perciformes: Pinguipedidae) off Brazil. J Parasitol 101:492–499. doi:10.1645/14-625.1
Ribeiro J, São Clemente SC, Lopes LMS, Knoff M (2014) Nematode larvae of hygienic importance infecting Chaetodipterus faber (Broussonet, 1782) and Trachinotus carolinus (Linnaeus, 1766) (Pisces: Teleostei) in Brazil. Rev Bras Med Vet 36:121–124
Shamsi S, Eisenbarth A, Saptarshi S, Beveridge I, Gasser RB, Lopata AL (2011) Occurrence and abundance of anisakid nematode larvae in five species of fish from southern Australian waters. Parasitol Res 108:927–934. doi:10.1007/s00436-010-2134-1
Shamsi S, Gasser R, Beveridge I (2013) Description and genetic characterization of Hysterothylacium (Nematoda: Raphidascarididae) larvae parasitic in Australian marine fishes. Parasitol Int 6:320–328. doi:10.1016/j.parint.2012.10.001
Shamsi S, Poupa A, Justine JL (2015) Characterization of Ascaridoid larvae from marine fish off New Caledonia, with description of new Hysterothylacium larval types XIII and XIV. Parasitol Int 54:397–404. doi:10.1016/j.parint.2015.05.014
Tavares LER, Luque JL (2006) Sistemática, Biologia e importância em saúde coletiva de larvas de Anisakidae (Nematoda: Ascaridoidea) parasitas de peixes ósseos marinhos do Estado do Rio de Janeiro, Brasil. In Sanidade de organismos aquáticos no Brasil, AT Silva-Souza, Abrapoa, Maringá, Paraná, Brasil, 387 p
Zhang L, Hu M, Shamsi S, Beveridge I, Li H, Xu Z, Li L, Cantacessi C, Gasser RB (2007) The specific identification of anisakid larvae from fishes from the Yellow Sea, China, using mutation scanning-coupled sequence analysis of nuclear ribosomal DNA. Mol Cell Probes 21:386–390. doi:10.1016/j.mcp.2007.05.004
Zhu X, Gasser RB, Podolska M, Chilton NB (1998) Characterization of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. Int J Parasitol 28:1911–1921. doi:10.1016/S0020-7519(98)00150-7
Acknowledgments
Camila S. Pantoja was supported by a Doctoral fellowship from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil), Felipe B. Pereira was supported by a Postdoctoral fellowship from PNPD-CAPES (Programa Nacional de Pós-Doutorado-Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), and José L. Luque was supported by a Researcher fellowship from CNPq. This study was also partially supported by FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro) and FAPERJ-BIOTA (E-26/112.563/2012). The authors would like to thank Dr. Douglas McIntosh from the Laboratory of Molecular Biology, Department of Animal Parasitology, UFRRJ, for technical support on sequencing of samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Pantoja, C.S., Pereira, F.B., Santos, C.P. et al. Morphology and molecular characterization hold hands: clarifying the taxonomy of Hysterothylacium (Nematoda: Anisakidae) larval forms. Parasitol Res 115, 4353–4364 (2016). https://doi.org/10.1007/s00436-016-5221-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5221-0