Abstract
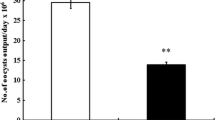
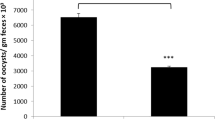
Eimeria spp. multiply within the intestinal tract causing severe inflammatory responses. Chitosan (CS), meanwhile, has been shown to exhibit anti-inflammatory activities in different experimental models. Here, we investigated the effect of CS on the outcome of inflammation caused by Eimeria papillata in the mouse intestine. Investigations were undertaken into the oocyst output in feces and developmental stages and goblet cells in intestinal tissue. Assays for lipid peroxidation, nitric oxide (NO), and myeloperoxidase (MPO) were also performed. T cells in intestinal tissue were counted using immunohistochemistry while total IgA in serum or intestinal wash was assayed using ELISA. In addition, mRNA expression of tumor necrosis factor alpha (TNF-α), transforming growth factor β (TGF-β), interleukin (IL)-10, and IL-4 were detected using real-time PCR. The data indicated a reduction in both oocyst output and in the number of parasite developmental stages following CS treatment, while the goblet cell hypoplasia in infected mice was also inhibited. CS decreased lipid peroxidation, NO, and MPO but did not alter the T cell count or IgA levels in comparison to the infected group. The expression of TNF-α and TGF-β decreased but IL-10 and IL-4 increased after CS treatment in comparison to the non-treated infected group. In conclusion, CS showed anti-inflammatory and protective effects against E. papillata infection.







Similar content being viewed by others
References
Al-Quraishy S, Delic D, Sies H, Wunderlich F, Abdel-Baki AS, Dkhil MA (2011) Differential miRNA expression in the mouse jejunum during garlic treatment of Eimeria papillata infections. Parasitol Res 109:387–394
Amer OS, Dkhil MA, Hikal WM, Al-Quraishy S (2015) Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. Biomed Res Int 2015:219670
Barocelli E, Ballabeni V, Ghizzardi P, Cattaruzza F, Bertoni S, Lagrasta CA, Impicciatore M (2006) The selective inhibition of inducible nitric oxide synthase prevents intestinal ischemia-reperfusion injury in mice. Nitric Oxide 14:212–218
Berkels R, Purol-Schnabel S, Roesen R (2004) Measurement of nitric oxide by reconversion of nitrate/nitrite to NO. Methods Mol Biol 279:1–8
Blanchard C, Durual S, Estienne M, Bouzakri K, Heim MH, Blin N, Cuber JC (2004) IL-4 and IL-13 up-regulate intestinal trefoil factor expression: requirement for STAT6 and de novo protein synthesis. J Immunol 172:3775–3783
Choi EH, Yang HP, Chun HS (2012) Chitooligosaccharide ameliorates diet-induced obesity in mice and affects adipose gene expression involved in adipogenesis and inflammation. Nutr Res 32:218–228
Delic D, Gailus N, Vohr HV, Dkhil M, Al-Quraishy S, Wunderlich F (2010) Testosterone-induced permanent changes of hepatic gene expression in female mice sustained during Plasmodium chabaudi malaria infection. J Mol Endocrinol 45:379–390
Dharmani P, Srivastava V, Kissoon-Singh V, Chadee K (2009) Role of intestinal mucins in innate host defense mechanisms against pathogens. J Innate Immun 1:123–135
Dkhil MA (2013) Anti-coccidial, anthelmintic and antioxidant activities of pomegranate (Punica granatum) peel extract. Parasitol Res 112:2639–2646
Dkhil MA, Al-Quraishy S, Abdel Moneim AE, Delic D (2013) Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol Res 112:101–106
Dkhil MA, Metwaly MS, Al-Quraishy S, Sherif NE, Delic D, Al Omar SY, Wunderlich F (2015) Anti-Eimeria activity of berberine and identification of associated gene expression changes in the mouse jejunum infected with Eimeria papillata. Parasitol Res 114:1581–1593
Fernandes J, Sereno J, Garrido P, Parada B, Cunha MF, Reis F, Pintado ME, Santos-Silva A (2012) Inhibition of bladder tumor growth by chitooligosaccharides in an experimental carcinogenesis model. Mar Drugs 10:2661–2675
Fernandes JC, Spindola H, de Sousa V, Park PJ, Shin ES, Sohn JH, Seo DB, Lim KM, Kim WG, Lee SJ (2010) Antiinflammatory activity of chitooligosaccharides in vivo. Mar Drugs 8:1763–1768
Gaafar MR, Mady RF, Diab RG, Shalaby TI (2014) Chitosan and silver nanoparticles: promising anti-toxoplasma agents. Exp Parasitol 143:30–38
Hierholzer C, Kalff JC, Billiar TR, Bauer AJ, Tweardy DJ, Harbrecht BG (2004) Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol 286:225–233
Hussain I, Singh T, Chittenden C (2012) Preparation of chitosan oligomers and characterization: their antifungal activities and decay resistance. Holzforschung 66:119–125
Inagaki-Ohara K, Dewi FN, Hisaeda H, Smith AL, Jimi F, Miyahira M, Abdel-Aleem AS, Horii Y, Nawa Y (2006) Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection. Infect Immun 74:5292–5301
Islam MA, Firdous J, Choi YJ, Yun CH, Cho CS (2012) Design and application of chitosan microspheres as oral and nasal vaccine carriers: an updated review. Int J Nanomedicine 7:6077–6093
Jakowlew SB, Mathias A, Lillehoj HS (1997) Transforming growth factor-beta isoforms in the developing chicken intestine and spleen: increase in transforming growth factor-beta 4 with coccidia infection. Vet Immunol Immunopathol 55:321–339
Khalil AM, Yasuda M, Farid AS, Desouky MI, Mohi-Eldin MM, Haridy M, Horii Y (2015) Immunomodulatory and antiparasitic effects of garlic extract on Eimeria vermiformis-infected mice. Parasitol Res 114:2735–2742
Kim JA, Ahn BN, Kong CS, Kim SK (2012) Chitooligomers inhibit UV-A-induced photoaging of skin by regulating TGF-beta/Smad signaling cascade. Carbohydr Polym 88:490–495
Kim MS, You HJ, You MK, Kim NS, Shim BS, Kim HM (2004) Inhibitory effect of water-soluble chitosan on TNF-alpha and IL-8 secretion from HMC-1 cells. Immunopharmacol Immunotoxicol 26:401–409
Li Y, Liu H, Xu QS, Du YG, Xu J (2014) Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-κB and endothelial inflammatory response. Carbohydr Polym 99:568–578
Lillehoj HS (1998) Role of T lymphocytes and cytokines in coccidiosis. Int J Parasitol 28:1071–1081
Linh BK, Hayashi T, Horii Y (2009) Eimeria vermiformis infection reduces goblet cells by multiplication in the crypt cells of the small intestine of C57BL/6 mice. Parasitol Res 104:789–794
Lüllau E, Heyse S, Vogel H, Marison I, von Stockar U, Kraehenbuhl JP, Corthésy B (1996) Antigen binding properties of purified immunoglobulin A and reconstituted secretory immunoglobulin A antibodies. J Biol Chem 271:16300–16309
McDougald LR (2003) Coccidiosis. In: Saif (ed) Diseases of poultry. Iowa State Press, Iowa, pp 1001–1010
Metwaly MS, Dkhil MA, Al-Quraishy S (2012) The potential role of Phoenix dactylifera on Eimeria papillata-induced infection in mice. Parasitol Res 111:681–687
Nam KS, Kim MK, Shon YH (2007) Chemopreventive effect of chitosan oligosaccharide against colon carcinogenesis. J Microbiol Biotechnol 17:1546–1549
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ohtsuka Y, Lee J, Stamm DS, Sanderson IR (2001) MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut 49:526–533
Ovington KS, Alleva LM, Kerr EA (1995) Cytokines and immunological control of Eimeria spp. Int J Parasitol 25:1331–1351
Porporatto C, Bianco ID, Correa SG (2005) Local and systemic activity of the polysaccharide chitosan at lymphoid tissues after oral administration. J Leukocyte Biol 78:62–69
Qiao Y, Bai XF, Du YG (2011) Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int Immunopharmacol 11:121–127
Schito ML, Barta JR, Chobotar B (1996) Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J Parasitol 82:255–262
Sun BW, Chen ZY, Chen X, Liu C (2007) Attenuation of leukocytes sequestration by carbon monoxide-releasing molecules: liberated carbon monoxide in the liver of thermally injured mice. J Burn Care Res 28:173–181
Tavaria FK, Soares JC, Reis IL, Paulo MH, Malcata FX, Pintado ME (2012) Chitosan: antimicrobial action upon staphylococci after impregnation onto cotton fabric. J Appl Microbiol 112:1034–1041
Tsakiris S, Schulpis KH, Marinou M, Behrakis P (2004) Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+, K+, -ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol Res 495:475–479
Prabhu VV, Guruvayoorappan C (2014) Protective effect of marine mangrove Rhizophora apiculata on acetic acid induced experimental colitis by regulating anti-oxidant enzymes, inflammatory mediators and nuclear factor-kappa B subunits. Int Immunopharmacol 18:124–134
Van der Lubben IM, Kersten G, Fretz MM, Beuvery C, Coos Verhoef J, Junginger HE (2003) Chitosan microparticles for mucosal vaccination against diphtheria: oral and nasal efficacy studies in mice. Vaccine 21:1400–1408
Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil MA (2014) Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol Res 113:3547–3556
Xiao D, Tang Z, Yin Y, Zhang B, Hu X, Feng Z, Wang J (2013) Effects of dietary administering chitosan on growth performance, jejunal morphology, jejunal mucosal sIgA, occludin, claudin-1 and TLR4 expression in weaned piglets challenged by enterotoxigenic Escherichia coli. Int Immunopharmacol 17:670–676
Xu JH, Dai WJ, Chen B, Fan XY (2015) Mucosal immunization with PsaA protein, using chitosan as a delivery system, increases protection against acute otitis media and invasive infection by Streptococcus pneumoniae. Scandinavian J Immunol 81:177–185
Yunus M, Horii Y, Makimura S, Smith AL (2005) Murine goblet cell hypoplasia during Eimeria pragensis infection is ameliorated by clindamycin treatment. J Vet Sci 67:311–315
Acknowledgments
We extend our appreciation to the Dean of Scientific Research, King Saud University, Riyadh, for funding the work through the research group project number RG -004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Abdel-Latif, M., Abdel-Haleem, H.M. & Abdel-Baki, AA.S. Anticoccidial activities of Chitosan on Eimeria papillata-infected mice. Parasitol Res 115, 2845–2852 (2016). https://doi.org/10.1007/s00436-016-5035-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5035-0