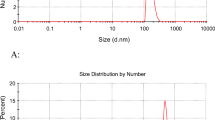
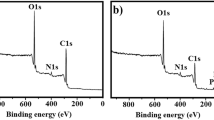
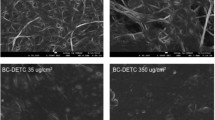
Abstract
Antileishmanial drugs traditionally used for treatment of cutaneous leishmaniasis are mainly toxic, ineffective for some parasite isolates, and costly. Since chitosan is reported to accelerate wound healing, the aims of this study were to assess the effectiveness of nanochitosan films in the treatment of cutaneous leishmaniasis caused by Leishmania major (Iranian strain) and to investigate alterations of antioxidant enzyme activities and malondialdehyde levels. Six weeks after L. major inoculation, when lesions appeared at the base of the tail of 36 female Balb/c mice, treatment was initiated. Lesion sizes, parasite proliferation, histopathological changes, antioxidant enzyme activities, and malondialdehyde changes were evaluated in groups treated with nanochitosan film and groups treated with the combination of nanochitosan film and daily peritoneal injection of glucantime. There was no significant difference between nanochitosan and glucantime in reduction of lesion size. But, lesion sizes in the group that was treated with a combination of nanochitosan film and glucantime were significantly reduced from 2 weeks after treatment. The results of the present study showed that application of nanochitosan film increased the wound contraction rate, reepithelialization rate, and scar tissue formation, and its combination with glucantime significantly reduced lesion size and parasite loads. Nanochitosan films alone or in combination with glucantime enhanced glutathione peroxidase (GPX) activity and decreased lipid peroxidation and oxidative stress. The results of this work support the potential usefulness of nanochitosan as a therapeutic agent against cutaneous leishmaniasis caused by L. major.



Similar content being viewed by others
References
Abdullah SM, Flath B, Presber W (1998) Mixed infection of human U-937 cells by two different species of Leishmania. Am J Trop Med Hyg 59:182–188
Akhtar F, Rizvi MM, Kar SK (2012) Oral delivery of curcumin bound to chitosan nanoparticles cured Plasmodium yoelii infected mice. Biotechnol Adv 30:310–320
Allahverdiyev AM, Abamor ES, Bagirova M, Ustundag CB, Kaya C, Kaya F, Rafailovich M (2011) Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light. Int J Nanomedicine 6:2705–2714
Badawy ME, El R, Rogge TM, Stevens CV, Smagghe G, Steurbaut W, Hofte M (2004) Synthesis and fungicidal activity of new N, O-acyl chitosan derivatives. Biogeosciences 5:589–595
Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A (2005) Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 26:6335–6342
Berman J (2003) Current treatment approaches to leishmaniasis. Curr Opin Infect Dis 16:397–401
Bivas-Benita M, Laloup M, Versteyhe S, Dewit J, De Braekeleer J, Jongert E, Borchard G (2003) Generation of Toxoplasma gondii GRA1 protein and DNA vaccine loaded chitosan particles: preparation, characterization, and preliminary in vivo studies. Int J Pharm 6:17–27
Chung Y, Su Y, Chen C, Jia G, Wang H, Wu J, Lin J (2004) Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol Sinica 25:932–936
Danesh-Bahreini MA, Shokri J, Samiei A, Kamali-Sarvestani E, Barzegar-Jalali M, Mohammadi-Samani S (2011) Nanovaccine for leishmaniasis: preparation of chitosan nanoparticles containing Leishmania superoxide dismutase and evaluation of its immunogenicity in BALB/c mice. Int J Nanomedicine 6:835–842
Du WL, Niu SS, Xu YL, Xu ZR, Fan CL (2009) Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carb Polym 75:385–389
Föger F, Noonpakdee W, Loretz B, Joojuntr S, Salvenmoser W, Thaler M, Bernkop-Schnürch A (2006) Inhibition of malarial topoisomerase II in Plasmodium falciparum by antisense nanoparticles. Int J Pharm 319:139–146
Guemouri L, Artur Y, Herbeth B (1991) Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin Chem 37:1932–1937
Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M (2006) Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med 3:e162
Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57:715–725
Howling GI, Dettmar PW, Goddard PA, Hampson FC, Dornish M, Wood EJ (2001) The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 22:2959–2966
Jafari M, Shirbazou S, Norozi M (2014) Induction of oxidative stress in skin and lung of infected BALB/C mice with Iranian strain of Leishmania major (MRHO/IR/75/ER). Iranian J Parasitol 9:60–69
Kayser O (2001) A new approach for targeting to Cryptosporidium parvum using mucoadhesive nanosuspensions. Res Appl 214:83–85
Koide SS (1998) Chitin-chitosan: properties, benefits and risks. Nutr Res 18:1091–1101
Kolodziejczyk L (1994) Study on glycogen content in the liver of mouse vs. pathomorphological changes in experimental fasciolosis. Acta Parasitol 39:211–216
Kumar R, Mishra AK, Dubey NK, Tripathi YB (2007) Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal activity, antiaflatoxigenic and antioxidant activity. Int J Food Microbiol 2:159–165
Leonardi D, Salomón CJ, Lamas MC, Olivieri AC (2009) Development of novel formulations for Chagas’ disease: optimization of benznidazole chitosan microparticles based on artificial neural networks. Int J Pharm 367:140–147
Lin SB, Chen SH, Peng KC (2009) Preparation of antibacterial chito-oligosaccharide by altering the degree of deacetylation of B-chitosan in a Trichoderma harzianumchitinase-hydrolysing process. J Sci Food Agric 89:238–244
Marcato PD, Duran N (2008) New aspects of nanopharmaceutical delivery systems. J Nanosci Nanotechnol 8:2216–2229
María GB, Leonardi D, Bolmaro RE, Claudia GE, Alejandro CO, Claudio JS, María CLI (2010) In vivo evaluation of albendazole microspheres for the treatment of Toxocara canis larva migrans. Eur J Pharm Biopharm 75:451–454
Mishra BB, Kale RR, Singh RK, Tiwari VK (2009) Alkaloids: future prospective to combat leishmaniasis. Fitoterapia 80:81–90
Momeni AZ, Aminjavaheri M (2003) Successful treatment of non-healing cases of cutaneous leishmaniasis, using a combination of meglumine antimoniate plus allopurinol. Eur J Dermatol 13:40–43
Muller RH, Jacobs C, Kayser O (2001) Nanosuspensions as particulate drug formulations in therapy rationale for development and what we can expect for the future. Adv Drug Delivery Rev 47:3–19
Muzzarelli RA, Guerrieri M, Goteri G, Muzzarelli C, Anneni T, Ghiselli R, Cornelissen M (2005) The biocompatibility of dibutyryl chitin in the context of wound dressings. Biomaterials 26:5844–5854
Oliveira CR, Rezende CMF, Silva MR, Olga M, Borges OM, Pêgo AP, Goes AM (2012) Oral vaccination based on DNA-chitosan nanoparticles against Schistosoma mansoni infection. Scientific World J 2012:1–11
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Placer ZA, Cushman L, Johnson B (1966) Estimation of product of lipid peroxidation (malonyldialdehyde) in biochemical system. Anal Biochem 16:359–364
Pujals G, Suñé-Negre JM, Pérez P, García E, Portus M, Tico JR, Miñarro M, Carrió J (2008) In vitro evaluation of the effectiveness and cytotoxicity of meglumine antimoniate microspheres produced by spray drying against Leishmania infantum. Parasitol Res 102:1243–1247
Rhim JW, Hong SI, Park HM, Ng PKW (2006) Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J Agric Food Chem 54:5814–5822
Romero FJ, Bosch-Morell F, Romero MJ, Jareno EJ, Romero B, Marin N, Roma J (1998) Lipid peroxidation products and antioxidants in human disease. Environ Health Perspect 106:1229–1234
Senel S, McClure SJ (2004) Potential applications of chitosan in veterinary medicine. Adv Drug Deliv Rev 56:1467–1480
Srivastava HC, Bhatt RM, Kant R, Yadav RS (2009a) Malaria associated with the construction of the Sardar Sarovar Project for water resources development, in Gujarat, India. Ann Trop Med Parasitol 103:653–658
Srivastava SK, Naresh B, Hema P (2009b) Traditional insect bioprospecting as human food and medicine. Ind J Tradit Knowl 8:485–494
Suzuki Y, Okamoto Y, Morimoto M, Sashiwa H, Saimoto H, Tanioka S, Shigemasa Y, Minami S (2000) Influence of physico-chemical properties of chitin and chitosan on complement activation. Carbohydr Polym 42:307–310
Ueno H, Nakamura F, Murakami M, Okumura M, Kadosawa T, Fujinaga T (2001) Evaluation effects of chitosan for the extracellular matrix production by fibroblasts and the growth factors production by macrophages. Biomaterials 22:2125–2130
Umakant S, Sarman S (2009) Immunobiology of leishmaniasis. Indian J Exp Biol 47:412–423
Upcroft P, Upcroft JA (2001) Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev 14:150–164
Wang X, Du Y, Liu H (2004) Preparation, characterization and antimicrobial activity of chitosan-Zn complex. Carbohydr Polym 56:21–26
Wochna A, Niemczyk E, Kurono C, Masaoka M, Majczak A, Kezior J, Slomiǹska E, Lipiǹski M, Wakabayashi T (2005) Role of mitochondria in the switch mechanism of the cell death mode from apoptosis to necrosis—studies on {rho} 0 cells. J Electron Microsc 54:127–138
World Health Organization (1991) Basic laboratory methods in medical parasitology. Report of a WHO Expert Committee
Xie W, Xu P, Liu Q (2001) Antioxidant activity of water-soluble chitosan derivatives. Bioorg Med Chem Lett 11:1699–1701
Acknowledgments
This study was supported by the research grant provided by Shahid Chamran University of Ahvaz.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 108 kb)
Rights and permissions
About this article
Cite this article
Bahrami, S., Esmaeilzadeh, S., Zarei, M. et al. Potential application of nanochitosan film as a therapeutic agent against cutaneous leishmaniasis caused by L. major . Parasitol Res 114, 4617–4624 (2015). https://doi.org/10.1007/s00436-015-4707-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4707-5