Abstract
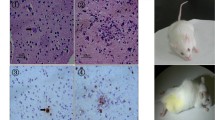
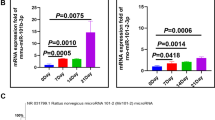
Angiostrongylus cantonensis (A. cantonensis) is a rodent nematode. Adult worms of A. cantonensis live in the pulmonary arteries of rats; humans are non-permissive hosts like the mice. The larva cannot develop into an adult worm and only causes serious eosinophilic meningitis or meningo-encephalitis if humans or mice eat food containing larva of A. cantonensis in the third stage. The differing consequences largely depend on differing immune responses of hosts to parasite during A. cantonensis invasion and development. To further understand the reasons why mice and rats attain different outcomes in A. cantonensis infection, we used the HE staining to observe the pathological changes of infected mice and rats. In addition, we measured mRNA levels of some cytokines (IL-5, IL-6, IL-13, Eotaxin, IL-4, IL-10, TGF-β, IFN-γ, IL-17A, TNF-α, IL-1β, and iNOS) in brain tissues of mice and rats by real-time PCR. The result showed that brain inflammation in mice was more serious than in rats. Meanwhile, mRNA expression levels of IL-6, IL-1β, TNF-α, and iNOS increased after mice were infected. In contrast, mRNA levels of these cytokines in rats brain tissues decreased at post- infection 21 days. These cytokines mostly were secreted by activated microglia in central nervous system. Microglia of mice and rats were showed by Iba-1 (microglia marker) staining. In micee brains, microglia got together and had more significant activation than in rats brains. The results demonstrate that mice and rats have different CNS inflammation after infection by A. cantonensis, and it is in line with other researchers’ reported findings. In conclusion, it is suggested that microglia activation is probably to be one of the most important factors in angiostrongyliasis from our study.





Similar content being viewed by others
References
Beaver PC, Rosen L (1964) Memorandum on the first report of Angiostrongylus in man, by Nomura and Lin, 1945. Am J Trop Med Hyg 13:589–590
Buchanan JB, Sparkman NL, Chen J, Johnson RW (2008) Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology 33:755–765
Chen KM, Lai SC (2007) Biochemical and pathological evaluation of albendazole/thalidomide co-therapy against eosinophilic meningitis or meningoencephalitis induced by Angiostrongylus cantonensis. J Antimicrob Chemother 59:264–276
Du WY, Liao JW, Fan CK, Su KE (2003) Combined treatment with interleukin-12 and mebendazole lessens the severity of experimental eosinophilic meningitis caused by Angiostrongylus cantonensis in ICR mice. Infect Immun 71:3947–353
Eamsobhana P, Yoolek A, Punthuprapasa P, Yong HS (2009) Thai koi-hoi snail dish and angiostrongyliasis due to Angiostrongylus cantonensis: effects of food flavoring and alcoholic drink on the third-stage larvae in infected snail meat. Foodborne Pathog Dis 6:401–405
Frank MG, Barrientos RM, Watkins LR, Maier SF (2010) Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol 226:181–184
He HJ, Lv ZY, Li ZY, Zhang LY, Liao Q, Zheng HQ, Su WY, Rao SQ, Yu XB, Wu ZD (2011) Efficacy of combined treatment with albendazole and baicalein against eosinophilic meningitis induced by Angiostrongylus cantonensis in mice. J Helminthol 85:92–99
Henry CJ, Huang Y, Wynne AM, Godbout JP (2009) Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun 23:309–317
Homsi S, Piaggio T, Croci N, Noble F, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M (2010) Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: a twelve-week follow-up study. J Neurotrauma 27:911–921
Hu X, Li JH, Lan L, Wu FF, Zhang EP, Song ZM, Huang HC, Luo FJ, Pan CW, Tan F (2012) In vitro study of the effects of Angiostrongylus cantonensis larvae extracts on apoptosis and dysfunction in the blood-brain barrier (BBB). PLoS One 7, e32161
Ishida K, Yoshimura K (1986) Eosinophil responses of permissive and nonpermissive hosts to the young adult worms of Angiostrongylus cantonensis. Parasitol Res 72:661–671
Kliks MM, Palumbo NE (1992) Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc Sci Med 34:199–212
Lai CH, Yen CM, Chin C, Chung HC, Kuo HC, Lin HH (2007) Eosinophilic meningitis caused by Angiostrongylus cantonensis after ingestion of raw frogs. Am J Trop Med Hyg 76:399–402
Lan KP, Lai SC (2009) Differences of proteolytic enzymes and pathological changes in permissive and nonpermissive animal hosts for Angiostrongylus cantonensis infection. Vet Parasitol 165:265–272
Lv S, Zhang Y, Steinmann P, Zhou XN (2008) Emerging angiostrongyliasis in Mainland China. Emerg Infect Dis 14:161–164
Raivich G (2005) Like cops on the beat: the active role of resting microglia. Trends Neurosci 28:571–573
Sawanyawisuth K (2008) Treatment of angiostrongyliasis. Trans R Soc Trop Med Hyg 102:990–996
Tsai HC, Chung LY, Chen ER, Liu YC, Lee SS, Chen YS, Sy CL, Wann SR, Yen CM (2008) Association of matrix metalloproteinase-9 and tissue inhibitors of metalloproteinase-4 in cerebrospinal fluid with blood-brain barrier dysfunction in patients with eosinophilic meningitis caused by Angiostrongylus cantonensis. Am J Trop Med Hyg 78:20–27
Tsai HC, Liu YC, Kunin CM, Lee SS, Chen YS, Lin HH, Tsai TH, Lin WR, Huang CK, Yen MY, Yen CM (2001) Eosinophilic meningitis caused by Angiostrongylus cantonensis: report of 17 cases. Am J Med 111:109–114
Tu WC, Lai SC (2006) Angiostrongylus cantonensis: efficacy of albendazole-dexamethasone co-therapy against infection-induced plasminogen activators and eosinophilic meningitis. Exp Parasitol 113:8–15
Wallace GD, Rosen L (1966) Studies on eosinophilic meningitis. 2. Experimental infection of shrimp and crabs with Angiostrongylus cantonensis. Am J Epidemiol 84:120–131
Wang J, Wei J, Zeng X, Liang JY, Wu F, Li ZY, Zheng HQ, He HJ, Wu ZD (2013) Efficacy of tribendimidine against Angiostrongylus cantonensis infection in the mice. Parasitol Res 112:1039–1046
Wang Z, Meng CJ, Shen XM, Shu Z, Ma C, Zhu GQ, Liu HX, He WC, Sun XB, Huo L, Zhang J, Chen G (2012) Potential contribution of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 to blood-brain barrier disruption and brain edema after experimental subarachnoid hemorrhage. J Mol Neurosci 48:273–280
Wei J, Wu F, Sun X, Zeng X, Liang JY, Zheng HQ, Yu XB, Zhang KX, Wu ZD (2013) Differences in microglia activation between rats-derived cell and mice-derived cell after stimulating by soluble antigen of IV larva from Angiostrongylus cantonensis in vitro. Parasitol Res 112:207–214
Yoshimura K (1993) Mechanism of parasite killing by eosinophils in parasitic infections. Nihon Rinsho 51:657–663
Yoshimura K, Sugaya H, Kawamura K, Kumagai M (1988) Ultrastructural and morphometric analyses of eosinophils from the cerebrospinal fluid of the mouse and guinea-pig infected with Angiostrongylus cantonensis. Parasite Immunol 10:411–423
Acknowledgments
This work was supported by National Natural Science Foundation of China (NSFC) (No: 81271855, 81261160324) to Z-D Wu and China Postdoctoral Science Foundation (No: 2014M552270) and NSFC (No: 81401686) to J Wei.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wei, J., Wu, F., He, A. et al. Microglia activation: one of the checkpoints in the CNS inflammation caused by Angiostrongylus cantonensis infection in rodent model. Parasitol Res 114, 3247–3254 (2015). https://doi.org/10.1007/s00436-015-4541-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4541-9