Abstract
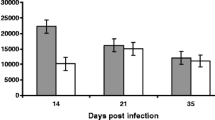
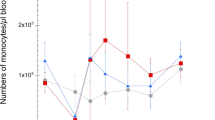
This histopathological study was carried out in order to investigate the cellular response in the jejunum to Ascaridia galli during the first 7 weeks of infection. Fourty-two ISA Brown chickens (7 weeks old) were infected orally with 500 embryonated A. galli eggs each while 28 chickens were left as uninfected controls. Six infected and four control chickens were necropsied at each time point 3, 7, 10, 14, 21, 28 and 42 days post-infection (dpi). Samples for histopathology were taken from three sites of the jejunoileum. Significantly higher eosinophil counts were seen in infected chickens compared to uninfected at 3, 7, 10, 14 and 28 dpi (P < 0.01). In both groups, the initial number of mast cells was high, but this high level of mast cells remained for a longer period in the infected group compared to the control group. Significantly higher counts were thus found in the infected group at 21 (P < 0.001), 28 (P < 0.01) and 42 dpi (P < 0.05). A. galli infection induced changes in the mucosal thickness as reduced villi length at 7, 10, 14, 21 and 28 dpi and in the degree of general cellular infiltration in the lamina propria of the mucosal layer. No adult worms were seen during the experiment; therefore, A. galli larvae have elicited a moderate cellular response in the lamina propria, mainly consisting of eosinophils in the early phase and later of mast cells.





Similar content being viewed by others
References
Ackert JE (1923) On the habitat of Ascaridia perspicillum (Rud.). J Parasitol 10:101–103
Ackert JE (1931) The morphology and life history of the fowl nematode Ascaridia lineate (Schneider). Parasitol 23:360–379
Ackert JE, Herrick CA (1928) Effects of the nematode Ascaridia lineata (Schneider) on growing chickens. J Parasitol 15:1–13
Ackert J, Porter D, Beach T (1935) Age resistance of chickens to the nematode Ascaridia lineate (Schneider). J Parasitol 21:205–213
Andreasen CB, Latimer KS (1990) Cytochemical staining characteristics of chicken heterophils and eosinophils. Vet Clin Pathol 19:51–54
Araujo P, Bressan C (1976) Observations on the second moult of the larvae of Ascaridia galli (author’s transl)]. Ann Parasitol Hum Comp 52:531–537
Araujo P, Bressan CR (1977) Observations on the second moult of the larvae of Ascaridia galli. Annales de Parasitologie Humaine et Comparee 52:531–537
Bancroft JD, Stevens A (1996) Theory and practice of histological techniques, 4th edn. Churchill Livingstone, New York, p 776
Dänicke S, Moors E, Beineke A, Gauly M (2009) Ascaridia galli infection of pullets and intestinal viscosity: consequences for nutrient retention and gut morphology. Br Poult Sci 50:512–520
Darmawi BU, Hambal PBP (2013) Mucosal mast cells response in the jejunum of Ascaridia galli-infected laying hens. Media-Peternakan J Animal Sci Tech 36(2):113–119
Degen WG, Nv D, Rothwell L, Kaiser P, Schijns VE (2005) Th1/Th2 polarization by viral and helminth infection in birds. Vet Microbiol 105:163–167
Ferdushy T, Nejsum P, Roepstorff A, Thamsborg SM, Kyvsgaard NC (2012) Ascaridia galli in chickens: intestinal localization and comparison of methods to isolate the larvae within the first week of infection. Parasitol Res 111:2273–2279
Ferdushy T, Luna-Olivares LA, Nejsum P, Roepstorff A, Thamsborg SM, Kyvsgaard NC (2013) Population dynamics of Ascaridia galli following single infection in young chickens. Parasitol 140:1078–1084
Galli SJ, Maurer M, Lantz CS (1999) Mast cells as sentinels of innate immunity. Curr Opin Immunol 11:53–59
Hansen M, Terhaar C, Turner D (1956) Importance of the egg shell of Ascaridia galli to the infectivity of its larva. J Parasitol 42:122–125
Herd RP, McNaught DJ (1975) Arrested development and the histotrophic phase of Ascaridia galli in the chicken. Int J Parasitol 5:401–406
Höglund J, Jansson DS (2011) Infection dynamics of Ascaridia galli in non-caged laying hens. Vet Parasitol 180:267–273
Ikeme M (1971) Observations on the pathogenicity and pathology of Ascaridia galli. Parasitol 63:169–179
Ishikawa N, Horii Y, Nawa Y (1993) Immune-mediated alteration of the terminal sugars of goblet cell mucins in the small intestine of Nippostrongylus brasiliensis-infected rats. Immunol 78:303
Jeurissen S, Janse E, Vermeulen A, Vervelde L (1996) Eimeria tenella infections in chickens: aspects of host-parasite: interaction. Vet Immunol Immunopathol 54:231–238
Jeurissen S, Wagenaar F, Janse EM (1999) Further characterization of M cells in gut-associated lymphoid tissues of the chicken. Poult Sci 78:965–972
Kaiser P (2007) The avian immune genome a glass half-full or half-empty. Cyt Gen Res 117:221–230
Karaca T, Yörük M, Uslu S (2006) Age‐related changes in the number of mast cells in the avian lymphoid organs. Anat Histol Embryol 35(6):375–379
Löscher T, Saathoff E (2008) Eosinophilia during intestinal infection. Best Prac Res Clin Gastr 22:511–536
Luna LG (1968) Manual of histological staining methods of the Armed Forces Institute of Pathology, 3rd edn. McGraw-Hill Book Company, New York, p 251
Luna-Olivares LA, Ferdushy T, Kyvsgaard NC, Nejsum P, Thamsborg SM, Roepstorff A, Iburg TM (2012) Localization of Ascaridia galli larvae in the jejunum of chickens 3 days post infection. Vet Parasitol 185:186–193
Marcos-Atxutegi C, Gandolfi B, Arangüena T, Sepulveda R, Arévalo M, Simon F (2009) Antibody and inflammatory responses in laying hens with experimental primary infections of Ascaridia galli. Vet Parasitol 161:69–75
Miquel N (2005) Immune responses in experimental Ascaris suum single infections in pigs. Ph.D. Thesis. The Royal Veterinary and Agricultural University. Copenhagen. Denmark
Pearse AGE (1985) Histochemistry. Theorical and applied. Analytical Technology. 4th ed. vol 2
Permin A, Bisgaard M, Frandsen F, Pearman M, Kold J, Nansen P (1999) Prevalence of gastrointestinal helminths in different poultry production systems. Br Poult Sci 40:439–443
Ramadan HH, Aboud znada NY (1992) Morphology and life history of Ascaridia galli in the domestic fowl that are raised in Jeddah. J KAU Sci 4:87–89
Reid WM, Carmon JL (1958) Effects of numbers of Ascaridia galli in depressing weight gains in chicks. J Parasitol 44:183–186
Schrank P Von F (1788) Verzeichniss der bisher hinlänglich bekannten Eingeweidewürmer, nebs einer Abhandlung über ihre Anverwandschaften. München, pp 116
Schummer A, Vollmerhaus B, Sinowatz F, Frewein J Waibl H (1992) Anatomie der Vögel. In Nickel, R., Schummer, A., Seiferle, E. (2.ed) Lehrbuch der Anatomie der Haustiere. Paul Parey, Berlin and Hamburg, Germany pp 203-204
Schwarz A, Gauly M, Abel H, Daş G, Humburg J, Rohn K, Breves G, Rautenschlein S (2011) Immunopathogenesis of Ascaridia galli infection in layer chicken. Dev Comparativ Immunol 35:774–784
Soulsby EJL (1982) Helminths, arthropods and protozoa of domesticated animals, 7th ed. Balliere Tindall. The English Language Book Society, London
Stenfeldt A, Wennerås C (2004) Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils. Immunol 112:605–614
Tugwell RL, Ackert JE (1952) On the tissue phase of the life cycle of the fowl nematode Ascaridia galli (Schrank). J Parasitol 38:277–288
Uribe C (1922) Observations on the development of Heterakis papillosa Bloch in the chicken. J Parasitol 8:167–176
Wells PD (1963) Mucin-secreting cells in rats infected with Nippostrongylus brasiliensis. Exp Parasitol 14:15–22
Xu L, Carr M, Bland A, Hall G (1993) Histochemistry and morphology of porcine mast cells. Histochem J 25:516–522
Yong L (1997) The mast cell: origin, morphology, distribution, and function. Exp Tox Path 49:409–424
Acknowledgements
DANIDA is thanked for financial support through grant DFC 09-054 LIFE. Thanks are given for the technical assistance from the Section of Pathology (Hanne Hornemann Møller, Betina Andersen and Lisbeth Kiørboe) and the Danish Centre for Experimental Parasitology (Lise-Lotte Christiansen), University of Copenhagen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luna-Olivares, L.A., Kyvsgaard, N.C., Ferdushy, T. et al. The jejunal cellular responses in chickens infected with a single dose of Ascaridia galli eggs. Parasitol Res 114, 2507–2515 (2015). https://doi.org/10.1007/s00436-015-4450-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4450-y