Abstract
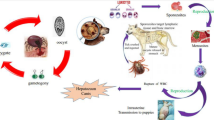
Encephalitozoon cuniculi is an obligate intracellular microsporidian parasite that infects a wide range of mammalian hosts. The present study investigated the prevalence of E. cuniculi in different animal hosts from different provinces of Egypt (Alexandria, Behera, and Assuit) using serological (IFAT and ELISA) and molecular (PCR) assays. A total of 324 serum and 274 urine samples were collected from seven different species of animals (cattle, buffaloes, sheep, goat, rabbit, dog, and rat). The results of serological examination confirmed the occurrence of antibodies against E. cuniculi in 38.9 % (126 out of 324) of the examined animals. The significant (P < 0.01) highest positivity was observed in goats (67 %) followed by buffaloes, rabbits, dogs, rat, and cattle (46.42, 41, 40, 36.2, and 28.1 %, respectively), while the least was recorded in sheep (9 %). Behera province showed the highest (P < 0.01) infection rate (40.68 %) followed by Alexandria and Assuit (39.2 and 22.73 %, respectively). The infection rate was significantly higher (P < 0.01) in females (45.34 %) than that in males (30.47 %). Positive cases were observed in all age categories. The highest infection rate (64.66 %) was recorded in the age group 1–5 years and the least was recorded in the age group <1 year (34.85 %).On the other hand, only five positive out of 274 urine samples (1.82 %) were detected by PCR. Our study provides a wide database on prevalence and epidemiology of an ignored parasite (E. cuniculi) for the first time in Egypt.
Similar content being viewed by others
References
Akerstedt J (2002) An indirect ELISA for detection of Encephalitozoon cuniculi infection in farmed blue foxes (Alopex lagopus). Acta Vet Scand 43:211–220
Akerstedt J (2003) Serological investigation of canine encephalitozoonosis in Norway. Parasitol Res 89:49–52
Akerstedt J, Kapel CM (2003) Survey for Encephalitozoon cuniculi in arctic foxes (Alopex lagopus) in Greenland. J Wildl Dis 39:228–232
Ashmawy KI, Abuakkada SS, Awad AM (2011) Seroprevalence of antibodies to Encephalitozoon cuniculi and Toxoplasma gondii in farmed domestic rabbits in Egypt. Zoonosis Public Health 58(5):357–364
Boot R, Hansen C, Nozari N, Thuis H (2000) Comparison of assays for antibodies to Encephalitozoon cuniculi in rabbits. Lab Anim 34(3):281–289
Bywater JEC, Kellett BS (1978) The eradication of Encephalitozoon cuniculi from a specific pathogen-free rabbit colony. Lab Anim Sci 28:402–404
Cislakova L, Literak I, Balent P, Hipikova V, Levkutova M, Travnicek M, Novotna A (2001) Prevalence of antibodies to Encephalitozoon cuniculi (Microsporida) in Angora goat—a potential risk of infection for breeders. Ann Agric Environ Med 8:289–291
Cox JC, Walden NB, Nairn RC (1972) Presumptive diagnosis of Nosema cuniculi in rabbits by immunofluorescence. Res Vet Sci 13:595–597
Cray C, Rivas Y (2013) Seroprevalence of Encephalitozoon cuniculi in dogs in the United States. J Parasitol 99(1):153–154
Csokai J, Joachim A, Gruber A, Tichy A, Pakozd A (2009) Diagnostic markers for encephalitozoonosis in pet rabbits. Vet Parasitol 163(1–2):18–26
Didier PJ, Didier ES, Snowden KS, Shadduck JA (1998) Encephalitozoonosis. In: Greene C (ed) Infectious diseases of the dog and cat. Saunders, Philadelphia, pp 465–470
Dipineto L, Rinaldi L, Santaniello A, Sensale M, Cuomo A, Calabria M, Menna LF, Fioretti A (2008) Serological survey for antibodies to Encephalitozoon cuniculi in pet rabbits in Italy. Zoonoses Public Health 55:173–175
Eroksuz Y, Eroksuz H, Ozer H, Cevik A, Unver O (1999) A survey of Encephalitozoon cuniculi infection in rabbit colonies in Elazig, Turkey: pathomorphologic and serologic (carbonimmunoassay test) studies. Isr J Vet Med 54:73–77
Ewringmann A, Göbel T (1999) Untersuchungen zur Klinik und Therapie der Encephalitozoonose beim Heimtierkaninchen. Kleintierpraxis 44:357–372
Furuya K (2009) Spore-forming microsporidian encephalitozoon: current understanding of infection and prevention in Japan. Jpn J Infect Dis 62:413–422
Ghatak S, Muthukumaran RB, Nachimuthu SK (2013) A simple method of genomic DNA extraction from human samples for PCR-RFLP analysis. J Biomol Tech 24(4):224–231
Ghosh K, Weiss LM (2009) Molecular diagnostic tests for microsporidia. Interdiscip Perspect Infect Dis, Article ID 926521: 1–13
Halánová M, Letková V, Macák V, Stefkovic M, Halán M (1999) The first finding of antibodies to Encephalitozoon cuniculi in cows in Slovakia. Vet Parasitol 82(2):167–171
Harcourt-Brown FM, Holloway HK (2003) Encephalitozoon cuniculi in pet rabbits. Vet Rec 152:427–431
Hersteinsson P, Gunnarsson ES, Hjartardottir S, Skirnisson K (1993) Prevalence of Encephalitozoon cuniculi antibodies in terrestrial mammals in Iceland, 1986 to 1989. J Wildlife Dis 29:341–344
Hilali M, Nassar AM, Ramadan EI (1991) Detection of encephalitozoonosis and toxoplasmosis among rabbits by carbon immunoassay. Vet Med J Giza 39:129–135
Hunt RD, King NW, Foster HL (1972) Encephalitozoonosis, evidence of verticaltransmission. J Infec Dis 126:212–214
Hůrková L, Modrý D (2006) PCR detection of Neospora caninum, Toxoplasma gondii andEncephalitozoon cuniculi in brains of wild carnivores. Vet Parasitol 137(1–2):150–154
Jeklova E, Leva L, Kovarcik K, Matiasovic J, Kummer V, Maskova J, Skoric M, Faldyna M (2010) Experimental oral and ocular Encephalitozoon cuniculi infection in rabbits. Parasitol 137(12):1749–1757
John RC (2009) The ELISA guidebook, 2nd ed Series: methods in molecular biology, pp 516–566
Jordan CN (2005) Encephalitozoon cuniculi: diagnostic test and methods of inactivation. MSc thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA
Jordan CN, Zajac AM, Lindsay DS (2006) Encephalitozoon cuniculi infection in rabbits. Compend Contin Educ Vet 28:108–116
Kasickova D, Sak B, Kvac M, Ditrich O (2009) Sources of potentially infectious human microsporidia: molecular characterization of microsporidia isolates from exotic birds in the Czech Republic, prevalence study and importance of birds in epidemiology of the human microsporidial infections. Vet Parasitol 165:125–130
Khan G, Kangro HO, Coates PJ, Heath RB (1991) Inhibitory effects of urine on the polymerase chain reaction for cytomegalovirus DNA. J Clinic Pathol 44:360–365
Künzel F, Joachim A (2010) Encephalitozoonosis in rabbits. Parasitol Res 106:299–309
Lindsay DS, Goodwin DG, Zajac AM, Corte´s-Vecino JA, Gennari SM, Rosypal AC, Dubey JP (2009) Serological survey for antibodies to Encephalitozoon cuniculi in ownerless dogs from urban areas of Brazil and Colombia. J Parasitol 95(3):760–763
Malcekova B, Halanova M, Sulinova Z, Molnár L, Ravaszova P, Adam J, Halán M, Valocky I, Baranovic M (2010) Seroprevalence of antibodies to Encephalitozoon cuniculi and Encephalitozoon intestinalis in humans and animals. ResVet Sci 89(3):358–361
Malik MA, Dressen DW, Cruz A (1990) Toxoplasmosis in sheep in North Eastern United States. J Am Vet Med Assoc 196:263–265
Mathis A, Weber R, Deplazes P (2005) Zoonotic potential of the microsporidia. Clin Microbiol Rev 18:423–445
Meyer-Breckwoldt A (1996) Epidemiological and clinical studies on encephalitozoonosis in dwarf rabbits. Thesis, Tierarztliche. Hochschule: Hannover, Germany
Muller-Doblies UU, Herzog K, Tanner I, Mathis A, Deplazes P (2002) First isolation and characterization of Encephalitozoon cuniculi from a free-ranging rat (Rattus norvegicus). Vet Parasitol 107(279):285
Murakoshi F, Fukuda Y, Matsubara R, Kato Y, Sato R, Sasaki T, Tada C, Nakai Y (2013) Detection and genotyping of Cryptosporidium spp. in large Japanese field mice, Apodemus speciosus. Vet Parasitol 196(1–2):184–188
Pakes SP, Shadduck JA, Feldman DB, Moore JA (1984) Comparison of tests for the diagnosis of spontaneous encephalitozoonosis in rabbits. Lab Anim Sci 34:356–359
Pye D, Cox JC (1977) Isolation of Encephalitozoon cuniculi from urine samples. Lab Anim 11:234
Reabel S (2012) Molecular diagnostic methods for detection of Encephalitozoon cuniculi in pet rabbits. Msc thesis in Pathobiology, University of Guelph, Guelph, Ontario, Canada
Reetz J (1995) Microsporidien als ursache von aborten beim rind. TieraÈrztl Umschau 50:550–554
Sak B, Kvac M, Kvetonova D, Albrecht T, Pialek J (2011) The first report on natural Enterocytozoon bieneusi and Encephalitozoon spp. infections in wild East European House Mice (Mus musculus musculus) and West-European House Mice (M. m. domesticus) in a hybrid zone across the Czech Republic Germany border. Vet Parasitol 178(3–4):246–250
SAS (2004) Statistical analysis system. SAS User’s Guide SAS Incorporation Institute
Sasaki M, Yamazaki A, Haraguchi A, Tatsumi M, Ishida K, Ikadai H (2011) Serological survey of Encephalitozoon cuniculi infection in Japanese dogs. J Parasitol 97(1):16–19
Schmidt EC, Shadduck JA (1983) Murine encephalitozoonosis model for studying the host parasite relationship of a chronic infection. Inf Immun 40:936–942
Schuurs AH, Verheul HA (1989) Effect of gender and steroids on the immune response. J Steroid Biochem 35:157–172
Soliman R, Selim S (2005) Practical immunology, 1st edn. Faculty of Veterinary Medicine, Cairo University Press, Egypt
Wasson K, Peper RL (2000) Mammalian microsporidiosis. Vet Pathol 37:113–128
Weber R, Bryan RT, Owen RL, Wilcox CM, Gorelkin NO, Visvesvara GS (1992) Improved light microscopical detection of microsporidia spores in stool and duodenal aspirates. N Engl J Med 326:161–166
Wilson K, Bjornstad ON, Dobson AP, Merler S, Poglayen G, Randolph SE, Read AF, Skorping A (2007) Heterogeneities in macroparasite infections: patterns and processes. In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP (eds) The ecology of wildlife disease. Oxford University Press, New York, pp 6–44
Yamaguchi-Shinozaki K, Koizumi M, Urao S, Shinozaki K (1992) Molecular cloning and characterization of one cDNAs for genes that are responsive to desiccation in Arabidopsis thaliana: sequence analysis of one cDNA clone that encodes a putative transmembrane channel protein. Plant Cell Physiol 33:217–224
Acknowledgments
The study was funded by the Alexandria University Research Fund (AURF), Grants, Innovation and Technology Transfer Center (GITTC), Research Enhancement Program (ALEX REP), 2011–2012 (project code, AGRV-11). The authors are indebted to Dr. Rafiq Soliman, professor of Microbiology, Faculty of Veterinary Medicine, Cairo University for his effort and valuable help.
Ethical standards
All investigations comply with the current laws of the countries in which they were performed.
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abu-Akkada, S.S., Ashmawy, K.I. & Dweir, A.W. First detection of an ignored parasite, Encephalitozoon cuniculi, in different animal hosts in Egypt. Parasitol Res 114, 843–850 (2015). https://doi.org/10.1007/s00436-014-4247-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4247-4