Abstract
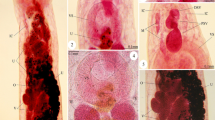
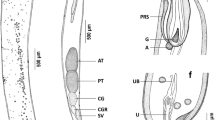
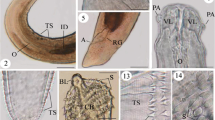
Seventy specimens of the gilthead sea bream Sparus aurata of the Red Sea were collected during the period from March to November 2013; they were dissected and examined for parasitic acanthocephalans. Only 40 (57.14 %) specimens were found to be naturally infected with Serrasentis sagittifer belonging to family Rhadinorhynchidae. The infection was recorded in the intestine, pyloric ceca, and the external surfaces of some internal organs of the infected fish. Seasonally, the prevalence of infection was increased to 77.14 % during summer season and decreased to 37.14 % during winter. Light and scanning electron microscopic investigation revealed that the adult worm was elongated (with broad anterior and narrow posterior ends) and measured 6.9–8.6 (7.6 ± 0.2) × 0.57–0.73 (0.63 ± 0.02) mm for male and 10.2–12.1 (11.5 ± 0.2) × 0.71–0.82 (0.76 ± 0.02) mm for female. Proboscis was long and cylindrical with a length of 0.97–1.6 mm (1.2 ± 0.2) for male and 1.12–1.17 mm (1.14 ± 0.02) for female. It was covered with numerous uniform spines arranged longitudinally as 9–11 rows each equipped by 15–18 spines. Spines were triangular, arrow-shaped, strong, and covered with cuticular theca; they decreased in size from the apex to the base of the proboscis. The proboscis is followed by a short spineless neck region followed by the body proper which is supported by multiple combs of spines (16–20) on its ventral surface. Molecular analysis of 18S rDNA sequence for the parasite demonstrated a close identity (>83 %) between the present acanthocephalan and other previously described species within class Palaeacanthocephala with 98 % identity with the previously recorded S. sagittifer (acc. no. JX014227) which is supported by the morphological data and the presence of trunk spines arranged within rows (comb-like) and the presence of four cement glands in the males. So, according to the records of morphological and molecular analyses, the present parasite is classified as S. sagittifer belonging to class Palaeacanthocephala and family Rhadinorhynchidae with a new host record from the gilthead sea bream S. aurata of the Red Sea.





Similar content being viewed by others
References
Ali N, Nazir S, Ganaie HA, Muzaffar G, Chishti MZ (2012) Prevalence studies of parasite Pomphorhynchus kashmirensis in local fish species Schizothorax. Eur Sci J 8(30):197–209
Alves DR, Paraguassú AR, Luque JL (2005) Community ecology of the metazoan parasites of the grey triggerfish, Balistes capriscus Gmelin, 1789 and queen triggerfish B. vetula Linnaeus, 1758 (Osteichthyes: Balistidae) from the State of Rio de Janeiro, Brazil. Rev Brasil de Parasitol Vet 14(2):71–77
Al-Zubaidy AB, Mhaisen FT (2012) A record of two species of Acanthocephala (Echinorhynchida: Rhadinorhynchidae) from Red Sea fishes, Yemeni coastal waters. Mesopot J Mar Sci 27(1):15–28
Amin OM (1987) Acanthocephalan from lake fishes in Wisconsin: ecology and host relationships of Pomphorhynchus bulbocoli (Pomphorhynchidae). J Parasitol 73(2):278–289
Amin OM, Nahhas FH, Al-Yamani F, Abu-Hakima R (1984) On three acanthocephalan species from some Arabian Gulf fishes off the coast of Kuwait. J Parasitol 70:168–170
Amin OM, Saoud MFA, Alkuwari KSR (2002) Neoechinorhynchus qatarensis sp. n. (Acanthocephala: Neoechinorhynchidae) from the blue-barred flame parrot fish, Scarus ghobban Forsskal, 1775, in Qatari waters of the Arabian Gulf. Parasitol Int 51(2):171–176
Amin OM, Evans P, Heckmann RA, El-Naggar AM (2013) The description of Mediorhynchus africanus n. sp. (Acanthocephala: Gigantorhynchidae) from galliform birds in Africa. Parasitol Res 112(8):2897–2906
Bilqees FM (1971) Marine fish, nematodes of West Pakistan. I. Description of seven new species of Karachi coast. J Sci Karachi 1:175–178
Bilqees FM, Khan A (1993) Paraechinorhynchus kalriai n. g, n. sp. (Neoechinorhynchidea: Neoechinorhynchinae) from Labeo rohita (Ham). Pakistan J Agric Sci 20(3&4):116–120
Brooks DR, McLennan DA (1993) Parascript: parasites and the language of evolution, Smithsonian Institution Press, Washington, D.C
Bullock WL (1969) Morphological features as tools and pitfalls in acanthocephalan systematics. In: Schmidt GD (ed) Problems in systematics of parasites. Univ. Park Press, Baltimore MD, pp 9–24
Bush AO, Lafferty KD, Lotz JM, Shostak W (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83(4):575–583
Datta MN (1954) On a new species of acanthocephalan parasites from fishes of Bombay. Rec Indian Mus 51:15–20
Dunagan TT, Miller DM (1991) Acanthocephala. In: Harrison FW, Rupert EE (eds) Microscopic anatomy of invertebrates, vol. 4: Aschelminthes. Wiley-Liss, New York, pp 299–332
Felsenstein J (1993) PHYLIP—Phylogeny Inference Package, version 3.5. University of Washington, Seattle
Froese R, Pauly D (Editors) (2011) FishBase. http://www.fishbase.org
Garcia-Varela M, Nadler S (2005) Phylogenetic relationships of Palaeacanthocephala (Acanthocephala) inferred from SSU and LSU rDNA gene sequences. J Parasitol 91:1401–1409
Garcia-Varela M, Cummings MP, Perez-Ponce de Leon G, Gardner SL, Lacletteam JP (2002) Phylogenetic analysis based on 18S ribosomal RNA gene sequences supports the existence of class Polyacanthocephala (Acanthocephala). Mol Phyl Evol 23:288–292
Garey JR, Near TJ, Nonnemacher MR, Nadler SA (1996) Molecular evidence for Acanthocephala as a subtaxon of Rotifera. J Mol Evol 43:287–292
Herlyn H, Piskurek O, Schmitz J, Ehlers U, Zischlera H (2003) The syndermatan phylogeny and the evolution of acanthocephalan endoparasitism as inferred from 18S rDNA sequences. Mol Phyl Evol 26:155–164
Integrated Taxonomic Information System (ITIS) (2011): http://www.itis.gov
Kardousha MM (2005) Helminth parasite larvae collected from Arabian Gulf fish. IV: Description of four larvae including two metacercariae, one didymozoid and one acanthocephalan from Emirati coast. Arab Gulf J Sci Res 23(1):23–27
Kumar S, Tamnra K, Nei M (1994) MEGA: molecular evolutionary genetics analysis software for microcomputers. Comput Appl Biosci 10:189–191
Kuzmina TA, Lisitsyna OI, Lyons ET, Spraker TR, Tolliver SC (2012) Acanthocephalans in northern fur seals (Callorhinus ursinus) and a harbor seal (Phoca vitulina) on St. Paul Island, Alaska: species, prevalence, and biodiversity in four fur seal subpopulations. Parasitol Res 111(3):1049–1058
Leidy J (1851) Descriptions of new species of Entozoa. Proc Acad Nat Sci Phila 5(7):155–156
Linnaeus C (1758) Systema Naturae, Ed. X. (Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus , differentiis, synonymis, locis. Tomus I. Editio decima, reformata). Holmiae. Systema Naturae, Ed. X. v. 1: i-ii + 1-824. [Nantes and Pisces in Tom. 1, pp. 230–338; a few species on later pages. Date fixed by ICZN, Code Article3]
Linton E (1889) Notes on Entozoa of marine fishes of New England. Ann Rep Comm Fish Wash 490pp., 2 ests
Luque JL, Poulin R (2004) Use of fish as intermediate hosts by helminth parasites: a comparative analysis. Acta Parasitol 49(4):353–361
Maghami SSG, Khanmohammadi M, Kerdeghari M (2008) Serrasentis sagittifer (Acanthocephala: Rhadinorhynchidae) from the Japanese thread fin bream, Nemipterus japonicus in Bushehr waters of Persian Gulf. J Anim Vet Adv 7(11):1430–1433
Martins ML, Moraes FR, Fujimoto RY, Onaka EM, Quintana CIF (2001) Prevalence and histopathology of Neoechinorhynchus curemai Noronha, 1973 (Acanthocephala: Neoechinorhynchidae) in Prochilodus lineatus Valenciennes, 1836 from Volta Grande Reservoir MG Brazil. Braz J Biol 61(3):517–522
Mehlhorn H, Taraschewski H, Franz M, Walldorf V, Voigt WP (1988) Reproduction. In: Mehlhorn H (ed) Parasitology in focus. Facts and trends. Springer, Berlin Heidelberg New York, pp 312–367
Meyers TR (1978) Prevalence of fish parasitism in Raritan Bay, New Jersey. Proc Helminthol Soc Wash 45(1):120–128
Radwan NA (2012) Phylogenetic analysis of Sphaerirostris picae (Acanthocephala: Centrorhynchidae) based on large and small subunit ribosomal DNA gene. Int J Parasitol Res 4(2):106–110
Salgado-Maldonado G (2013) Redescription of Neoechinorhynchus (Neoechinorhynchus) golvani Salgado-Maldonado, 1978 (Acanthocephala: Neoechinorhynchidae) and description of a new species from freshwater cichlids (Teleostei: Cichlidae) in Mexico. Parasitol Res 112(5):1891–1901
Sanil NK, Asokan PK, John L, Vijayan KK (2010) Pathological manifestations of the acanthocephalan parasite, Tenuiproboscis sp. in the mangrove red snapper (Lutjanus argentimaculatus) (Forsskål, 1775), a candidate species for aquaculture from Southern India. Aqua 310:259–266
Santos CP, Gibson DI, Tavares LER, Luque JL (2008) Checklist of Acanthocephala associated with the fishes of Brazil. Zootaxa 1938:1–22
Seyed SGM, Majid K, Mahnaz K (2008) Serrasentis sagittifer (Acanthocephala: Rhadinorhynchidae) from the Japanese thread fin bream, Nemipterus japonicus, in Bushehr water of Persian Gulf. J Anim Vet Adv 7(11):1430–1433
Taraschewski H (2000) Host-parasite interactions in Acanthocephala: a morphological approach. Adv Parasitol 46:1–179
Taraschewski H (2005) Acanthocephala (thorny or spiny-headed worms). In: Rhode K (ed) Marine parasitology. CSIRO Publishing, Collingwood, pp 116–121
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nuc A Res 24:4876–4882
Tingbao Y, Xianghua L (2001) Seasonal population dynamics of Neoechinorhynchus quinghaiensis in the carp, Gymnocypris przewalskii przewalskii from Qinghai Lake, China. J Helminthol 75:93–98
Travassos L (1923) Informações sobre a fauna helminthologica de Matto Grosso (II nota). Folha Medica 4:12
Tripathi YR (1959) Studies on parasites of Indian fishes. V. Acanthocephala. Rec Indian Mus 54(1/2):61–99
Van Cleave HJ (1923) Telosentis, a new genus of Acanthocephala from southern Europe. J Parasitol 9:174–175
Van de Peer Y, Van den Broeck I, De Rijk R, De Wachter R (1994) Database on the structure of small ribosomal subunit RNA. Nucleic Acids Res 22:3488–3494
Verweyen L, Klimpel S, Palm HW (2011) Molecular phylogeny of the Acanthocephala (Class Palaeacanthocephala) with a paraphyletic assemblage of the orders Polymorphida and Echinorhynchida. PLoS ONE 6(12):e28285
Vignon M, Sasal P (2010) The use of geometric morphometrics in understanding shape variability of sclerotized haptoral structures of monogeneans (Platyhelminthes) with insights into biogeographic variability. Parasitol Int 59:183–191
Wang PQ (1981) Some digenetic trematodes of marine fishes from Fujian Province, China. Wuyi Sci J 2:65–74
World Register of Marine Species (WoRMS) (2011) http://www.marinespecies.org
Yamaguti S (1963) Systema Helminthum, vol. 5. Acanthocephala. Wiley Interscience, New York and London, p 423
Acknowledgments
This work is supported by Faculty of Science, Cairo University, Cairo, Egypt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Ghaffar, F., Morsy, K., Abdel-Gaber, R. et al. Prevalence, morphology, and molecular analysis of Serrasentis sagittifer (Acanthocephala: Palaeacanthocephala: Rhadinorhynchidae), a parasite of the gilthead Sea bream Sparus aurata (Sparidae). Parasitol Res 113, 2445–2454 (2014). https://doi.org/10.1007/s00436-014-3889-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3889-6