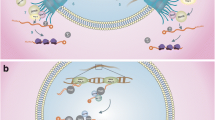
Abstract
Several proteins and different species of RNA that are produced in the nucleus are exported through the nuclear pore complexes, which require a family of conserved nuclear export receptors called exportins (XPOs). It has been reported that the XPOs (XPO1, XPO5, and XPOT) are directly involved in the transport processes of noncoding RNAs from the nucleus to the cytoplasm and/or from cytoplasm to the nucleus. All three genes are present in fungi, plants, and deuterostome metazoans. However, protostome metazoan species lack one of the three genes across evolution. In this report, we have demonstrated that all three XPO proteins are present in the parasite protostome Schistosoma mansoni. As this parasite has a complex life cycle presenting several stages in different hosts and environments, implying a differential gene regulation, we proposed a genomic analysis of XPOs to validate their annotation. The results showed the conservation of exportin family members and gene duplication events in S. mansoni. We performed quantitative RT-PCR, which revealed an upregulation of SmXPO1 in 24 h schistosomula (sixfold when compared with cercariae), and similar transcription levels were observed for SmXPO5 and SmXPOT in all the analyzed stages. These three XPO proteins have been identified for the first time in the protostome clade, which suggests a higher complexity in RNA transport in the parasite S. mansoni. Taken together, these results suggest that RNA transport by exportins might control cellular processes during cercariae, schistosomula, and adult worm development.



Similar content being viewed by others
References
Arts GJ, Kuersten S, Romby P, Ehresmann B, Mattaj IW (1998) The role of exportin-t in selective nuclear export of mature tRNAs. EMBO J 17:7430–7441
Basch P, DiConza J (1977) In vitro development of Schistosoma mansoni cercariae. J Parasitol 63:245–249
Berriman M, Protasio AV, Dunne DW (2013) Comparative study of transcriptome profiles of mechanical and transformed Schistosoma mansoni schistosomula. PloS Negl Trop Dis 7:e2091
Blanton RE, Licate LS (1992) Developmental regulation of protein synthesis in schistosomes. Mol Biochem Parasitol 51:201–208
Bohnsack MT, Regener K, Schwappach B, Saffrich R, Paraskeva E, Hartmann E, Görlich D (2002) Exp5 exports eEF1A via tRNA from nuclei and synergizes with other transport pathways to confine translation to the cytoplasm. EMBO J 21:6205–6215
Bussing I, Yang JS, Lai EC, Grosshans H (2010) The nuclear export receptor XPO-1 supports primary miRNA processing in C. elegans and Drosophila. EMBO J 29:1830–1839
Calado A, Treichel N, Muller EC, Otto A, Kutay U (2002) Exportin‑5‑mediated nuclear export of eukaryotic elongation factor 1A and tRNA. EMBO J 21:6216–6224
Castanotto D, Lingeman R, Riggs AD, Rossi JJ (2009) CRM1 mediates nuclear-cytoplasmic shuttling of mature microRNAs. Proc Natl Acad Sci USA 106:21655–21659
Cook AG, Fukuhara N, Jinek M, Conti E (2009) Structures of the tRNA export factor in the nuclear and cytosolic states. Nature 46:60–65
Fried H, Kutay U (2003) Nucleocytoplasmic transport: taking an inventory. Cell Mol Life Sci 60:1659–1688
Gomes MS, Cabral FJ, Jannotti-Passos LK, Carvalho O, Rodrigues V, Baba EH, Sá RG (2009) Preliminary analysis of miRNA pathway in Schistosoma mansoni. Parasitol Int 58:61–68
Han ZG, Brindley PJ, Wang SY, Chen Z (2009) Schistosoma genomics: new perspectives on schistosome biology and host–parasite interaction. Annu Rev Genomics Hum Genet 10:211–240
Harrop R, Wilson RA (1993) Protein synthesis and release by cultured schistosomula of Schistosoma mansoni. Parasitology 107:265–274
Hellmuth K, Lau DM, Bischoff FR, Kunzler M, Hurt E, Simos G (1998) Yeast Los1p has properties of an exportin-like nucleocytoplasmic transport factor for tRNA. Mol Cell Biol 18:6374–6386
Hutten S, Kehlenbach RH (2007) CMR1-mediated nuclear export: to the pore and beyond. Trends Cell Biol 17:193–201
Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10:126–139
Köhler A, Hurt E (2007) Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol 8:761–773
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Lacker DH, Bahler J (2008) Chapter 5 translational control of gene expression: from transcripts to transcriptomes. Int Rev Cell Mol Biol 271:199–251
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425:415–419
Leisegang MS, Martin R, Ramírez AS, Bohnsack MT (2012) Exportin t and Exportin 5: tRNA and miRNA biogenesis—and beyond. Biol Chem 393:599–604
Lipowsky G, Bischoff FR, Izaurralde E, Kutay U, Schäfer S, Gross HJ, Beier H, Görlich D (1999) Coordination of tRNA nuclear export with processing of tRNA. RNA 5:539–549
Lipowsky G, Bischoff FR, Schwarzmaier P, Kraft R, Kostka S, Hartmann E, Kutay U, Görlich D (2000) Exportin 4: a mediator of a novel nuclear export pathway in higher eukaryotes. EMBO J 19:4362–4371
Liu S, Cai P, Hou N, Piao X, Wang H, Hung T, Chen Q (2012) Genome-wide identification and characterization of a panel of house-keeping genes in Schistosoma japonicum. Mol Biochem Parasitol 182:75–82
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta DeltaC(T)) Method. Methods 25:402–408
Lund E, Dahlberg JE (1998) Proofreading and aminoacylation of tRNAs before export from the nucleus. Science 282:2082–2085
Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U (2004) Nuclear export of microRNA precursors. Science 303:95–98
Mingot JM, Bohnsack MT, Jakle U, Gorlich D (2004) Exportin 7 defines a novel general nuclear export pathway. EMBO J 23:3227–3236
Murphy D, Dancis B, Brown JR (2008) The evolution of core proteins involved in microRNA biogenesis. BMC Evol Biol 8:92
Nagai Y, Gazzinelli G, De Moraes GWG, Pellegrino J (1977) Protein synthesis during cercaria schistosomulum transformation and early development of the Schistosoma mansoni larvae. Comp Biochem Physiol B 57:27–30
Nakielny S, Dreyfuss G (1999) Transport of proteins and RNAs in and out of the nucleus. Cell 99:677–690
Nguyen KT, Holloway MP, Altura RA (2012) The CMR1 nuclear export protein in normal development and disease. Int J Biochem Mol Biol 3:137–151
Nilsen TM (2008) Endo-siRNAs: yet another layer of complexity in RNA silencing. Nat Struct Mol Biol 15:546–548
Perron MP, Provost P (2008) Protein interactions and complexes in human microRNA biogenesis and function. Front Biosci 13:2537–2547
Protasio AV, Tsai IJ, Babbage A, Nichol S, Hunt M, Aslett MA, Silva N, Velarde GS, Anderson TJC, Clark RC, Davidson C, Dillon GP, Holroyd NE, LoVerde PT, Lloyd C, McQuillan J, Oliveira G, Otto TD, Parker-Manuel SJ, Quail MA, Wilson RA, Zerlotini A, Dunne DW, Berriman M (2012) A systematically improved high quality genome and transcriptome of the human blood fluke Schistosoma mansoni. PLoS Negl Trop Dis 6:e1455
Rodriguez MS, Dargemont C, Françoise S (2004) Nuclear export of RNA. Biol Cell 96:639–655
Saito K, Ishizuka A, Siomi H, Siomi MC (2005) Processing of pré-microRNAs by the Dicer-1-Loquacious complex in Drosophila cells. PLoS Biol 3:e235
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schütz S, Chemnitz J, Spillner C, Frohme M, Hauber J, Kehlenbach RH (2006) Stimulated expression of mRNAs in activated T cells depends on a functional CRM1 nuclear export pathway. J Mol Biol 358:997–1009
Song J, Joshua-Tor L (2006) Argonaute and RNA—getting into the groove. Curr Opin Struck Biol 16:5–11
Stuven T, Hartmann E, Gorlich D (2003) Exportin 6: a novel nuclear export receptor that is specific for profilin actin complexes. EMBO J 22:5928–5940
Yi R, Qin W, Macara IG, Cullen BR (2003) Exportin-5 mediates the nuclear export pre-microRNAs and short hairpin RNAs. Genes Dev 17:3011–3016
Yi R, Doehle BP, Qin Y, Macara IG, Cullen BR (2005) Overexpression of Exportin 5 enhances RNA interference mediated by short hairpin RNAs and microRNAs. RNA 11:220–226
Yuckenberg PD, Poupin F, Mansour TE (1987) Schistosoma mansoni: protein composition and synthesis during early development: evidence for early synthesis of heat shock proteins. Exp Parasitol 63:301–311
Acknowledgments
The authors thank the transcriptome initiatives: São Paulo Transcriptome Consortium, Minas Gerais Genome Network, and Wellcome Trust Genome Initiative (UK). This work was supported by the following Brazilian research agencies: Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, CBB 0558/09) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(JPEG 38 kb)
Rights and permissions
About this article
Cite this article
Abreu, F.C.P., Pereira, R.V., Oliveira, V.F. et al. Characterization of export receptor exportins (XPOs) in the parasite Schistosoma mansoni . Parasitol Res 112, 4151–4159 (2013). https://doi.org/10.1007/s00436-013-3606-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3606-x