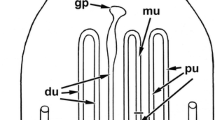
Abstract
In the first study of the vaginal ultrastructure of any monogenean, the paired vaginae of Chimaericola leptogaster, a basal polyopisthocotylean from the gills of a holocephalan, are described. Each vagina opens laterally. A unique feature of this relict parasite is a short vagino-intestinal connecting duct, the lining of which is separated by septate junctions from the linings of the vagina and the intestine. After giving rise to this vagino-intestinal duct, each vagina travels in a posterior direction and opens into the vitelline collecting duct. The lining of each vagina close to the vaginal pore resembles the body tegument, the syncytial lining of which measures about 2.5 μm in thickness and has a dense surface layer 0.2 μm in thickness and different apical projections. Proximal to this and before the entrance to the vaginal-intestinal duct, the vaginal lining is characterised by: uniformly distributed electron-dense, lanceolate, spine-like surface structures of about 1.7 μm in length; three kinds of surrounding glands with three kinds of secretory granules (abundant oval or rounded electron-dense granules 0.7–2.5 μm in length, much less numerous spheroidal, vesicular granules 0.3–0.4 μm in diameter, and tightly packed, spheroidal granules 0.8 μm in diameter and containing fine particulate material of low density); and four kinds of sensory receptors (three uniciliate and one aciliate). These features are absent from the rest of the vagina. The likely roles of the vagino-intestinal connection, the different gland cells, sensory structures, and the armament of the distal vaginal regions are discussed. Considering the suggested polyopisthocotylean ancestor for the Neodermata, the relict parasite fauna of holocephalans and the unique vagino-intestinal connection in C. leptogaster, which is also known in turbellarians, an ancient origin for chimaericolids is supported.





Similar content being viewed by others
References
Andersen K (1975) Ultrastructural studies on Diphyllobothrium ditremum and D. dendriticum (Cestoda, Pseudophyllidea), with emphasis on the scolex tegument and the tegument in the area around the genital atrium. Parasitol Res 46:253–264. doi:10.1007/BF00418519
Beveridge I, Smith K (1985) An ultrastructural study of the cirrus and vagina of Phyllobothrium vagans (Cestoda: Tetraphyllidea). Parasitol Res 71:609–616. doi:10.1007/BF00925594
Beverley-Burton M, Chisholm LA, Allison FR (1993) The species of Callorhynchicola Brinkmann (Monogenea: Chimaericolidae) from Callorhinchus spp. (Chimaeriformes: Callorhinchidae): adult morphology and the larval haptor. Syst Parasitol 24:201–215. doi:10.1007/BF00010533
Boeger WA, Kritsky DC (1993) Phylogeny and a revised classification of the Monogenoidea Bychowsky, 1937 (Platyhelminthes). Syst Parasitol 26:1–32. doi:10.1007/BF00009644
Boeger WA, Kritsky DC (1997) Coevolution of the Monogenoidea (Platyhelminthes) based on a revised hypothesis of parasite phylogeny. Int J Parasitol 27:1495–14511. doi:10.1016/S0020-7519(97)00140-9
Boeger WA, Kritsky DC (2001) Phylogenetic relationships of the Monogenoidea. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor & Francis, London, UK, pp 92–102
Brinkmann A Jr (1952a) Fish trematodes from Norwegian water. The history of fish trematode investigations in Norway and the Norwegian species of the order Monogenea Arbok for Universitetet i Bergen. Naturvitenskapelig rekke 1:1–74
Brinkmann A Jr (1952b) Some Chilean monogenetic trematodes. Kungliga Fysiografiska Sällskapets i Lund. Förhandlingar 62:3–26
Brinkmann A Jr (1942). On “Öctobothrium” leptogaster F.S. Leuckart. Göteborgs Kungl. Vetenskaps och Vitterhets Samhälles Handlingar, Ser. B 2: 3–29
Brooks DR (1989) The phylogeny of the Cercomeria (Platyhelminthes: Rhabdocoela) and general evolutionary principles. J Parasitol 75:606–616
Bychowsky BE (1957) Monogenetic trematodes, their systematics and phylogeny. Izdatel’stvo Academiya Nauk SSSR, Moscow, Russia, In Russian
El-Naggar MM, Arafa SZ, El-Abbassy SA, Steward MT, Halton DW (2007) Neuromusculature of Macrogyrodactylus congolensis, a monogenean skin parasite of the Nile catfish Clarias gariepinus. Parasitol Res 100:265–279
Euzet L, Combes C (2003) Some controversial questions regarding Monogenea. In: Combes C, Jourdane J (eds) Taxonomy, ecology and evolution of metazoan parasites. Vol. 1. Presses Universitaires de Perpignan, Perpignan, France, pp 303–320
Gibson DI, Bray RA (1979) The Hemiuroidea: terminology, systematics and evolution. Bull Brit Mus Nat Hist (Zool) 36:35–146
Gremigni V (1983) Platyhelminthes—Turbellaria. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates. Vol. 1. Oogenesis, oviposition and oosorption. John Wiley & Sons Ltd, Chichester, UK, pp 67–107
Gustafsson MKS, Halton DW, Kreshchenko ND, Movsessian SO, Raikova OI, Reuter M, Terenina NB (2002) Neuropeptides in flatworms. Peptides 23:2053–2061. doi:10.1016/S0196-9781(02)00193-6
Halton DW, Hardcastle A (1977) Ultrastructure of the male accessory ducts and prostate gland of Diclidophora merlangi (Monogenoidea). Int J Parasitol 7:393–401. doi:10.1016/0020-7519(77)90065-0
Halton DW, Morris GP (1969) Occurrence of cholinesterase and ciliated sensory receptors in a fish gill fluke, Diclidophora merlangi (Trematoda; Monogenea). Parasitol Res 33:21–30. doi:10.1007/BF00259567
Halton DW, Brennan GP, Maule AG, Shaw C, Johnston CF, Fairweather I (1991) The ultrastructure and immunogold labelling of pancreatic polypeptide-immunoreactive cells associated with the egg-forming apparatus of a monogenean parasite, Diclidophora merlangi. Parasitology 102:429–436. doi:10.1017/S003118200006404
Inoue JG, Miya M, Lam K, Tay BH, Danks JA, Bell J, WalkerTI VB (2010) Evolutionary origin and phylogeny of the modern holocephalans (Chondrichthyes: Chimaeriformes): a mitogenomic perspective. Mol Biol Evol 27:2576–2586. doi:10.1093/molbev/msq147
Jovelin R, Justine J-L (2001) Phylogenetic relationships within the polyopisthocotylean monogeneans (Platyhelminthes) inferred from partial 28S rDNA sequences. Int J Parasitol 31:393–401. doi:10.1016/S0020-7519(01)00114-X
Kearn GC (1986) The eggs of monogeneans. Adv Parasitol 25:175–273. doi:10.1016/S0065-308X(08)60344-9
Kearn GC, Evans-Gowing R (1998) Attachment and detachment of the anterior adhesive pads of the monogenean (platyhelminth) parasite Entobdella soleae from the skin of the common sole (Solea solea). Int J Parasitol 28:1583–1593. doi:10.1016/S0020-7519(98)00059-9
Kearn GC, Whittington ID, Evans-Gowing R (1995) Use of cement for attachment in Neocalceostomoides brisbanensis, a calceostomatine monogenean from the gill chamber of the blue catfish, Arius graeffei. Int J Parasitol 25:299–306. doi:10.1016/0020-7519(94)00142-B
Littlewood DTJ, Rohde K, Clough KA (1999) The interrelationships of all major groups of Platyhelminthes: phylogenetic evidence from morphology and molecules. Biol J Linn Soc 66:75–114. doi:10.1111/j.1095-8312.1999.tb01918.x
Llewellyn J (1965) The evolution of parasitic platyhelminths. In: Taylor A (ed) Evolution of parasites. Third symposium of the British Society for Parasitology. Blackwell, Oxford, UK, pp 47–78
Llewellyn J, Simmons JE (1984) The attachment of the monogenean parasite Callorhynchicola multitesticulatus to the gills of its holocephalan host Callorhynchus milii. Int J Parasitol 14:191–196. doi:10.1016/0020-7519(84)90048-1
Maule AG, Halton DW, Johnston CF, Shaw C, Fairweather I (1990) A cytochemical study of the serotoninergic, cholinergic and peptidergic components of the reproductive system in the monogenean parasite Diclidophora merlangi. Parasitol Res 76:409–419. doi:10.1007/BF00933549
Ogawa K (1997) Copulation and egg production of the monogenean Heterobothrium okamotoi, a gill parasite of cultured tiger puffer (Takifugu rubripes). Fish Pathol 32:219–223. doi:10.3147/jsfp.32.219
Pascoe PL (1997) Monogenean parasites of deep-sea fishes from the Rockall Trough (N.E. Atlantic) including a new species. J Mar Biol Ass UK 67:603–622. doi:10.1017/S0025315400027326
Perkins EM, Donnellan SC, Bertozzi T, Whittington ID (2010) Closing the mitochondrial circle on paraphyly of the Monogenea (Platyhelminthes) infers evolution in the diet of parasitic flatworms. Int J Parasitol 40:1237–1245. doi:10.1016/j.ijpara.2010.02.0171
Poddubnaya LG (2002) Ultrastructure of the reproductive ducts of Diphyllobothrium latum (Cestoda, Pseudophyllidea) males. Zool Zh 81:394–405 (In Russian)
Rohde K (1972) Ultrastructure of the nerves and sense receptors of Polystomoides renschi Rohde and P. malayi (Rohde) (Monogenea: Polystomatidae). Parasitol Res 40:307–320. doi:10.1007/BF00328749
Rohde K (1973) Ultrastructure of the caecum of Polystomoides malayi Rohde and P. renschi Rohde (Monogenea: Polystomatidae). Int J Parasitol 3:461–466. doi:10.1016/0020-7519(73)90040-4
Rohde K, Ebrahimzadeh A (1969) Das weibliche Geschlechtssystem der Gattung Polystomoides Ward, 1917 (Monogenea). Parasitol Res 33:110–134. doi:10.1007/BF00259512
Rohde K, Watson NA (1995) Ultrastructure of the buccal complex of Polylabroides australis (Monogenea, Polyopisthocotylea, Microcotylidae). Int J Parasitol 3:307–318. doi:10.1016/0020-7519(94)00083-Z
Rohde K, Watson NA (1996) Ultrastructure of the buccal complex of Pricea multae (Monogenea: Polyopisthocotylea, Gastrocotylidea). Folia Parasitol 43:117–132
Tyler S (1976) Comparative ultrastructure of adhesive systems in the Turbellaria. Zoomorphol 84:1–76. doi:10.1007/BF0256855
Whittington ID, Cribb BW (1999) Morphology and ultrastructure of the anterior adhesive areas of the capsalid monogenean parasites Benedenia rohdei from the gills and B. lutjani from the pelvic fins of Lutjanus carponotatus (Pisces: Lutjanidae). Parasitol Res 85:399–408. doi:10.1007/s004360050566
Whittington ID, Cribb BW (2001) Adhesive secretions in the Platyhelminthes. Adv Parasitol 48:101–223. doi:10.1016/S0065-308X(01)48006-7
Whittington ID, Armstrong WD, Cribb BW (2004) Mechanism of adhesion and detachment at the anterior end of Neoheterocotyle rhinobatidis and Troglocephalus rhinobatidis (Monogenea: Monopisthocotylea: Monocotylidae). Parasitol Res 94:91–95. doi:10.1007/s00436-004-1171-z
Wong W-L, Brennan GP, Halton DW, Maule AG, Lim LHS (2008) Secretory products of the haptoral reservoirs and peduncular glands in two species of Bravohollisia (Monogenean: Ancyrocephalidae). Inverteb Biol 127:139–152. doi:10.1111/j.1744-7410.2007.00118.x
Acknowledgments
Special thanks are due to Prof. Odd Halvorsen, Natural History Museum, Oslo University, Norway for his kind assistance with finding a source of chimaeras. The authors would like to thanks the staff of the RV ‘Johan Ruud’, belonging to Tromsø University, for their invaluable help with the fishing. We are also grateful to the staff of the Centre of Electron Microscopy of the I.D. Papanin Institute of the Biology for Inland Waters, RAS, for technical assistance. The present study was supported by the Russian Foundation for Fundamental Research project no. 12-04-00149a (to LGP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poddubnaya, L.G., Hemmingsen, W. & Gibson, D.I. Ultrastructural characteristics of the vaginae of the basal monogenean Chimaericola leptogaster (Leuckart, 1830). Parasitol Res 112, 4053–4064 (2013). https://doi.org/10.1007/s00436-013-3596-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3596-8