Abstract
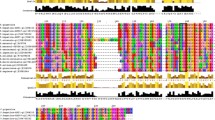
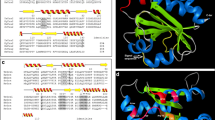
In this work, we studied a recombinant mu-class glutathione transferase of 25.5 kDa from Taenia solium metacestode (rTs25GST1-1) that follows Michaelis–Menten kinetics with 1-chloro-2,4-dinitrobenzene (CDNB). The kinetic parameters obtained for rTs25GST1-1 with CDNB and GSH were V max = 12.04 μmol/min/mg and Km = 1.38 mM, and V max = 10.20 μmol/min/mg and Km = 0.90, respectively. The optimal activity was found at pH 8 in the 37–40 °C temperature range. Circular dichroism studies for rTs25GST1-1 at different pH showed that it maintains a typical α-helix structure between pH 6.5–7.5, but loses it between pH 8 and 8.5. Thermal CD assays showed rTs25GST1-1 barely changed its secondary structure. Unfolding/refolding assays showed that rTs25GST1-1 retained its structure up to 40 °C without loss of its activity. Additionally, exposure of rTs25GST1-1 to cumene hydroperoxide did not produce significant changes in its structure and only affected 50 % of its activity.






Similar content being viewed by others
References
Andersson C, Mosialou E, Weinander R, Hebert H, Morgenstern R (1993) Rat liver microsomal glutathione transferase: studies on structure and function. In: Tew TW, Pickett CB, Mantle TJ, Mannervik B, Hayes JD (eds) Structure and function of glutathione transferases. CRC Press Inc, Boca Raton, pp 109–116
Armstrong RN (1994) Glutathione S-transferases: structure and mechanism of an archetypical detoxification enzyme. Adv Enzymol 69:1–44
Armstrong RN (1997) Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol 10(1):2–18
Board PG, Coggan M, Watson S, Gage PW, Dulhunty AF (2004) CLIC-2 modulates cardiac ryanodine receptor Ca2+ release channels. Int J Biochem Cell Biol 36(8):1599–1612
Böhn G, Muhr R, Jaenicke R (1992) Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Eng 5(3):191–5
Brophy PM, Pritchard DI (1994) Parasitic helminth glutathione S-transferases: an update on their potential as targets for immuno- and chemotherapy. Exp Parasitol 79(1):89–96
Cho SG, Lee YH, Park HS, Ryoo K, Kang KW, Park J, Eom SJ, Kim MJ, Chang TS, Choi SY, Shim J, Kim Y, Dong MS, Leei MJ, Kim SG, Ichijo H, Choi EJ (2001) Glutathione S-Transferase Mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem 276:12749–12755
Dirr HW, Wallace LA (1999) Role of the C-Terminal Helix 9 in the stability and ligandin function of class r glutathione transferase A1-1. Biochemistry 38:15631–15640
Eaton DL, Bammler TK (1999) Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol Sci 49(2):156–164
Frova C (2006) Glutathione transferases in the genomics era: new insights and perspectives. Biomol Eng 23:149–169
Girotti AW (1998) Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res 39:1529–1542
Habig HW, Jacoby WB (1981) Assays for differentiation of glutathione S-transferases. Meth Enzym 77:398–405
Halliwell B, Gutteridge J (2007) Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death. In: Halliwell B, Gutteridge J (eds) Free radicals in biology and medicine. Oxford University Press, USA, pp 187–267
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88
Hill BG, Bhatnagar A (2011) Protein S-glutathiolation: redox-sensitive regulation of protein function. J Mol Cell Cardiol 52(3):559–567
Hornby J, Luo J-K, Stevens JM, Wallace LA, Kaplan W, Armstrong RN, Dirr HW (2000) Equilibrium folding of dimeric class μ glutathione transferases involves a stable monomeric intermediate. Biochemistry 39:12336–12344
Jakobsson PJ, Morgenstern R, Mancini J, Ford-Hutchinson A, Persson B (1999) Common structural features of MAPEG-A widespread superfamily of membrane associated proteins with highly divergent functions in eicosanoid and glutathione metabolism. Prot Sci 8:689–692
Ji X, Zhang P, Armstrong RN, Gilliland GL (1992) The three-dimensional structure of a glutathione S-transferase from the mu gene class. Structural analysis of the binary complex of isoenzyme 3–3 and glutathione at 2.2-A resolution. Biochemistry 31(42):10169–10184
Johnson WC (1990) Protein secondary structure and circular dichroism: a practical guide. Proteins 7:205–214. doi:10.1002/prot.340070302
Liebau E, Wildenburg G, Brophy PM, Walter RD, Henkle-Dürsen K (1996) Molecular cloning, expression and characterization of a recombinant glutathione S-transferase from Echinococcus multilocularis. Mol Biochem Parasitol 80:27–39
Mannervik B (1985) The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol 57:357–417
Nguyen HA, Bae YA, Lee EG, Kim SH, Diaz-Camacho SP, Nawa Y, Kang I, Kong Y (2010) A novel sigma-like glutathione transferase of Taenia solium metacestode. Int J Parasitol 40:1097–1106
Oakley AJ (2005) Glutathione transferases: new functions. Curr Opin Struct Biol 15:716–723
Reinemer P, Dirr HW, Ladenstein R, Schäffer J, Gallay O, Huber R (1991) The three-dimensional structure of class pi glutathione S-transferase in complex with glutathione sulfonate at 2.3 A resolution. EMBO J 10(8):1997–2005
Reinemer P, Dirr HW, Ladenstein R, Huber R, Lo Bello M, Federici G, Parker MW (1992) Three-dimensional structure of class pi glutathione S-transferase from human placenta in complex with S-hexylglutathione at 2.8 A resolution. J Mol Biol 227(1):214–226
Rossjohn J, McKinstry WJ, Oakley AJ, Parker MW, Stenberg G, Mannervik B, Dragani B, Cocco R, Aceto A (2000) Structures of thermolabile mutants of human glutathione transferase P1-1. J Mol Biol 302(2):295–302
Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360:1–16
Sinning I, Kleywegt GJ, Cowan SW, Reinemer P, Dirr HW, Huber R, Gilliland GL, Armstrong RN, Ji X, Board PG (1993) Structure determination and refinement of human alpha class glutathione transferase A1-1, and a comparison with the Mu and Pi class enzymes. J Mol Biol 232(1):192–212
Slaughter RL, Edwards DJ (1995) Recent advances: the cytochrome P450 enzymes. Ann Pharmacother 29(6):619–624
Sluis-Cremer N, Naidoo N, Dirr H (1996) Class-pi glutathione S-transferase is unable to regain its native conformation after oxidative inactivation by hydrogen peroxide. Eur J Biochem 242:301–307
Tew KD, Manevich Y, Grek C, Xiong Y, Uys J, Townsend DM (2011) The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic Biol Med 51:299–313
Torres-Rivera A, Landa A (2008) Cooperative kinetics of the recombinant glutathione transferase of Taenia solium and characterization of the enzyme. Arch Biochem Biophys 477:372–378
Vibanco-Pérez N, Jimenez L, Mendoza-Hernández G, Landa A (2002) Characterization of a recombinant mu-class glutathione S-transferase form Taenia solium. Parasitol Res 88:398–404
Warholm M, Guthenberg C, Mannervik B (1983) Molecular and catalytic properties of glutathione transferase p from human liver: an enzyme efficiently conjugating epoxides. Biochemistry 22:3610–3617
Wu Y, Fan Y, Xue B, Luo L, Shen J, Zhang S, Jiang Y, Yin Z (2006) Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene 25:5787–5800
Xiong Y, Manevich Y, Tew KD, Townsend DM (2012) S-glutathionylation of protein disulfide isomerase regulates estrogen receptor α stability and function. Int J Cell Biol. doi:10.1155/2012/273549
Acknowledgments
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT-176925) and Dirección General de Asuntos del Personal Académico (DGAPA-PAPIIT IN 219711). Aramis Roldan was supported by CONACyT (231036) and is a student of Posgrado en Ciencias Biológicas (PCBIOL) of Universidad Nacional Autónoma de Mexico (UNAM). Authors declare that the experiments to produce antibodies in rabbits comply with the current laws for animal use and care (NOM-062-ZOO-1999) of Mexico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roldan, A., Torres-Rivera, A. & Landa, A. Structural and biochemical studies of a recombinant 25.5 kDa glutathione transferase of Taenia solium metacestode (rTs25GST1-1). Parasitol Res 112, 3865–3872 (2013). https://doi.org/10.1007/s00436-013-3577-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3577-y