Abstract
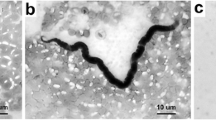
The mosquito midgut is the first site that vector-borne pathogens contact during their multiplication, differentiation, or migration from blood meal to other tissues before transmission. After blood feeding, the mosquitoes synthesize a chitinous structure called peritrophic matrix (PM) that envelops the blood meal and separates the food bolus from the midgut epithelium. In this study, a systematic investigation of the PM formation and the interaction of Brugia malayi within the midgut of a susceptible vector, Ochlerotatus togoi, were performed using scanning electron microscopy (SEM). SEM analysis of the midguts dissected at different time points post feeding on a B. malayi-infected blood meal (PIBM) revealed that the PM was formed from 45 min PIBM and gradually thickened and matured during 8–18 h PIBM. The PM degraded from 24 to72 h PIBM, when digestion was completed. The invasion process of the microfilariae was observed between 3 and 4 h PIBM. In the beginning of the process, only sheathed microfilariae interacted with the internal face of the PM by its anterior part, and then the midgut epithelium before entering the hemocoel, after that they exsheathed. Microfilarial sheaths lying within the hemocoel were observed suggesting that they may serve as a decoy to induce the immune systems of the mosquitoes to respond to the antigens on the sheaths, thereby protecting the exsheathed microfilariae. These initial findings would lead to further study on the proteins, chemicals, and factors in the midgut that are involved in the susceptibility of O. togoi as a vector of filariasis.





Similar content being viewed by others
References
Agudelo-Silva F, Spielman A (1985) Penetration of mosquito midgut wall by sheathed microfilariae. J Invertebr Pathol 45:117–119
Bain O, Brengues J (1972) Transmission of wuchereriasis and of bovine setariasis: histological study of the passage of microfilariae through the stomach wall of Anopheles gambiae and Aedes aegypti. Ann Parasitol Hum Comp 47:399–412
Bain O, Philippon B (1969) Mechanism of passing microfilaria through the stomach wall of a vector: its importance in onchocerciasis. C R Acad Sci Hebd Seances Acad Sci D 269:1081–1083
Bangs MJ, Ash LR, Barr AR (1995) Susceptibility of various mosquitoes of California to subperiodic Brugia malayi. Acta Trop 59:323–332
Billingsley PF (1994) Approaches to vector control: new and trusted. 2. Molecular targets in the insect midgut. Trans R Soc Trop Med Hyg 88:136–140
Blackburn K, Wallbanks KR, Molyneux DH, Lavin DR, Winstanley SL (1988) The peritrophic membrane of the female sandfly Phlebotomus papatasi. Ann Trop Med Parasitol 82:613–619
Chen CC, Shih CM (1988) Exsheathment of microfilariae of Brugia pahangi in the susceptible and refractory strains of Aedes aegypti. Ann Trop Med Parasitol 82:201–206
Cheun HI, Cho SH, Lee HI, Shin EH, Lee JS, Kim TS, Lee WJ (2011) Seasonal prevalence of mosquitoes, including vectors of Brugian filariasis, in southern islands of the Republic of Korea. Korean J Parasitol 49:59–64
Chang MS, Chan KL, Ho BC (1991) Comparative transmission potential of three Mansonia mosquitoes (Diptera: Culicidae) for filariasis in Sarawak, Malaysia. Bull Entomol Res 81:437–444
Chomcharn Y, Surathin K, Bunnag D, Sucharit S, Harinasuta T (1980) Effect of a single dose of primaquine on a Thai strain of Plasmodium falciparum. Southeast Asian J Trop Med Public Health 11:408–412
Choochote W, Chaithong U, Somboon P, Pakdicharoen A, Tookyang B, Likitvong K, Siriprasert V, Sukontasan K, Thitasut P (1991) Small laboratory animal model for nocturnally subperiodic Brugia malayi (Narathiwat province, southern Thailand strain). J Trop Med Parasitol 14:51–58
Choochote W, Keha P, Sukhavat K, Khamboonruang C, Sukontason K (1987) Aedes (Finlaya) togoi Theobald 1907, Chanthaburi strain. A laboratory vector in studies of filariasis in Thailand. Southeast Asian J Trop Med Public Health 18:259–260
Choochote W, Sucharit S, Abeywickreme W (1983) A note on adaptation of Anopheles annularis Van Der Wulp, Kanchanaburi, Thailand to free mating in a 30 × 30 ×30 cm cage. Southeast Asian J Trop Med Public Health 14:559–560
Choochote W, Sukhavat K, Somboon P, Khamboonruang C, Maleewong W, Suwanpanit P (1986) The susceptibility of small laboratory animals to nocturnally superiodic Brugia malayi in Thailand. J Parasitol Trop Med Assoc Thailand 9:35–37
Christensen BM, Sutherland DR (1984) Brugia pahangi: exsheathment and midgut penetration in Aedes aegypti. Trans Amer Microscop Soc 4:423–433
Crampton JM, Warren A, Lycett GJ, Hughes MA, Comley IP, Eggleston P (1994) Genetic manipulation of insect vectors as a strategy for the control of vector-borne disease. Ann Trop Med Parasitol 88:3–12
Di Luca M, Romi R, Severini F, Toma L, Musumeci M, Fausto AM, Mazzini M, Gambellini G, Musumeci S (2007) High levels of human chitotriosidase hinder the formation of peritrophic membrane in anopheline vectors. Parasitol Res 100:1033–1039
Esslinger JH (1962) Behavior of microfilaria of Brugia pahangi in Anopheles quadrimaculatus. AmJTrop Med Hyg 11:749–758
Freyvogel T, Staubli W (1965) The formation of the peritrophic membrane in Culicidae. Acta Trop 22:118–147
Guptavanij P, Harinasuta C, Vutikes S, Deesin T, Surathin K (1978) The vectors of Brugia malayi in southern Thailand. Southeast Asian J Trop Med Public Health 9:543–548
Gwadz RW (1994) Genetic approaches to malaria control: how long the road? AmJTrop Med Hyg 50:116–125
Hegedus D, Erlandson M, Gillott C, Toprak U (2009) New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol 54:285–302
Houk EJ, Obie F, Hardy JL (1979) Peritrophic membrane formation and the midgut barrier to arboviral infection in the mosquito, Culex tarsalis Coquillett (Insecta, Diptera). Acta Trop 36:39–45
Iyengar MOT (1936) Entry of filaria larvae into the body cavity of the mosquito. Parasitol 28:190–195
Jacobs-Lorena M, Oo MM (1996) The peritrophic matrix of insects. In: Beaty J, Marquardt WC (eds) The biology of disease vectors. University Press of Colorado, Boulder, pp 318–332
Kumar NP, Sabesan S, Panicker KN (1998) Susceptibility status of Mansonia annulifera to Brugia malayi parasites in Cherthala, Alappuzha district, Kerala. Indian J Exp Biol 36:829–831
Laurence BR (1966) Intake and migration of the microfilariae of Onchocerca volvulus (Leuckart) in Simulium damnosum Theobald. J Helminthol 50:337–342
Laurence BR, Pester FRN (1961) The behavior and development of Brugia patei (Buckley, Nelson and Heisch, 1958) in a mosquito host, Mansonia uniformis (Theobald). J Helminthol 35:285–300
Lehane MJ (1997) Peritrophic matrix structure and function. Annu Rev Entomol 42:525–550
Lek-Uthai U, Tomoen W (2005) Susceptibility of Mansonia uniformis to Brugia malayi microfilariae from infected domestic cat. Southeast Asian J Trop Med Public Health 36:434–441
Luo H, Qu FY (1990) Experimental infection index of Anopheles sinensis and melanization of periodic Brugia malayi. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 8:260–263
Meis JF, Ponnudurai T (1987) Ultrastructural studies on the interaction of Plasmodium falciparum ookinetes with the midgut epithelium of Anopheles stephensi mosquitoes. Parasitol Res 73:500–506
Meis JF, Pool G, van Gemert GJ, Lensen AH, Ponnudurai T, Meuwissen JH (1989) Plasmodium falciparum ookinetes migrate intercellularly through Anopheles stephensi midgut epithelium. Parasitol Res 76:13–19
O’Connor FW, Beatty H (1936) The early migrations of Wuchereria bancrofti in Culex fatigans. Trans R Soc Trop Med Hyg 30:125–127
Okuda K, Caroci A, Ribolla P, Marinotti O, de Bianchi AG, Bijovsky AT (2005) Morphological and enzymatic analysis of the midgut of Anopheles darlingi during blood digestion. J Insect Physiol 51:769–776
Pascoa V, Oliveira PL, Dansa-Petretski M, Silva JR, Alvarenga PH, Jacobs-Lorena M, Lemos FJA (2002) Aedes aegypti peritrophic matrix and its interaction with heme during blood digestion. Insect Biochem Mol 32:517–523
Perrone JB, Spielman A (1986) Microfilarial perforation of the midgut of a mosquito. J Parasitol 72:723–727
Perrone JB, Spielman A (1988) Time and site of assembly of the peritrophic membrane of the mosquito Aedes aegypti. Cell Tissue Res 252:473–478
Peters W (1992) Peritrophic membranes. In: Bradshaw D, Burggren W, Heller HC, Ishii S, Langer H, Neuweiler G, Randall DJ (eds) Zoophysiology. Springer-Verlag, Berlin, pp 1–238
Petit G (1978) The filaria Dipetalonema dessetae: phenomena of regulation and parasite yield in the Aedes vector. Ann Parasitol Hum Comp 53:649–668
Petit G, Spitalier-Kaveh H (1979) The Filaria Breinlia booliati in the Aedes togoi adipose tissue; comparison with the couple Dipetalonema dessetae-A. aegypti. Ann Parasitol Hum Comp 54:653–663
Ramachandran CP, Wharton RH, Dunn FL, Kershaw WE (1963) Aedes (Finlaya) togoi Theobold, a useful laboratory vector in studies of filariasis. Ann Trop Med Parasitol 57:443–445
Sadlova J, Volf P (2009) Peritrophic matrix of Phlebotomus duboscqi and its kinetics during Leishmania major development. Cell Tissue Res 337:313–325
Sanders HR, Evans AM, Ross LS, Gill SS (2003) Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect Biochem Mol Biol 33:1105–1122
Santos JN, Lanfredi RM, Pimenta PF (2006) The invasion of the midgut of the mosquito Culex (Culex) quinquefasciatus Say, 1823 by the helminth Litomosoides chagasfilhoi Moraes Neto, Lanfredi and De Souza, 1997. J Invertebr Pathol 93:1–10
Sasa M (1976) Human filariasis: a global survey of epidemiology and control. Tokyo. University of Tokyo Press, Tokyo, p 819
Schacher JF (1962) Morphology of the microfilaria of Brugia pahangi and of the larval stages in the mosquito. J Parasitol 48:679–692
Secundino NFC, Eger-Mangrich I, Braga EM, Santoro MM, Pimenta PFP (2005) Lutzomyia longipalpis peritrophic matrix: formation, structure, and chemical composition. J Med Entomol 42:928–938
Shahabuddin M, Cociancich S, Zieler H (1998) The search for novel malaria transmission-blocking targets in the mosquito midgut. Parasitol Today 14:493–497
Shen Z, Jacobs-Lorena M (1997) Characterization of a novel gut-specific chitinase gene from the human malaria vector Anopheles gambiae. J Biol Chem 272:28895–28900
Shih CM, Chen CC (1987) Exsheathment of microfilariae of Brugia pahangi in Anopheles quadrimaculatus and Culex quinquefasciatus. Southeast Asian J Trop Med Public Health 18:521–525
Singh RN, Rathaur S (2003) Setaria cervi: in vitro released collagenases and their inhibition by Wuchereria bancrofti infected sera. J Helminthol 77:77–81
Smartt CT, Chiles J, Lowenberger C, Christensen BM (1998) Biochemical analysis of a blood meal-induced Aedes aegypti glutamine synthetase gene. Insect Biochem Mol Biol 28:935–945
Syafruddin AR, Kamimura K, Kawamoto F (1991) Penetration of the mosquito midgut wall by the ookinetes of Plasmodium yoelii nigeriensis. Parasitol Res 77:230–236
Tellam RL, Wijffels G, Willadsen P (1999) Peritrophic matrix proteins. Insect Biochem Mol Biol 29:87–101
Terra WR (2001) The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch Insect Biochem Physiol 47:47–61
Trpis M (1981) Susceptibility of the autogenous group of the Aedes scutellaris complex of mosquitoes to infection with Brugia malayi and Brugia pahangi. Tropenmed Parasitol 32:184–188
Villalon JM, Ghosh A, Jacobs-Lorena M (2003) The peritrophic matrix limits the rate of digestion in adult Anopheles stephensi and Aedes aegypti mosquitoes. J Insect Physiol 49:891–895
Wada Y (2011) Vector mosquitoes of filariasis in Japan. Trop Med Health 39:39–45
Walters LL, Irons KP, Guzman H, Tesh RB (1993) Formation and composition of the peritrophic membrane in the sand fly, Phlebotomus perniciosus (Diptera: Psychodidae). J Med Entomol 30:179–198
Wu Y, Preston G, Bianco AE (2008) Chitinase is stored and secreted from the inner body of microfilariae and has a role in exsheathment in the parasitic nematode Brugia malayi. Mol Biochem Parasitol 161:55–62
Yamamoto H, Ogura N, Kobayashi M, Chigusa Y (1983) Studies on filariasis II: exsheathment of the microfilariae of Brugia pahangi in Armigeres subalbatus. Jpn J Parasitol 32:287–292
Zhu Z, Gern L, Aeschlimann A (1991) The peritrophic membrane of Ixodes ricinus. Parasitol Res 77:635–641
Acknowledgments
This work was financially supported by the Thailand Research Fund (TRF Senior Research Scholar: RTA5480006 to WC, subproject to NJ) and the Faculty of Medicine, Chiang Mai University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jariyapan, N., Saeung, A., Intakhan, N. et al. Peritrophic matrix formation and Brugia malayi microfilaria invasion of the midgut of a susceptible vector, Ochlerotatus togoi (Diptera: Culicidae). Parasitol Res 112, 2431–2440 (2013). https://doi.org/10.1007/s00436-013-3404-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3404-5