Abstract
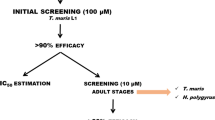
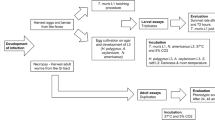
The present study investigates the in vitro efficacy of derivatives of the cyclooctadepsipeptides and the aminophenylamidines, which are promising candidates for the evaluation of the treatment of human soil-transmitted helminthiases. The effects of emodepside and PF1022A as well as of amidantel, deacylated amidantel and tribendimidine were evaluated in a concentration range between 0.01 and 100 μg/ml against third-stage larvae (L3) and adult worms of Nippostrongylus brasiliensis and first-stage larvae (L1) of Trichinella spiralis. Furthermore, drug combinations of PF1022A plus deacylated amidantel or tribendimidine and of tribendimidine plus levamisole were tested for any potential additive or even synergistic interactions. Emodepside had a significantly lower EC50 value than PF1022A in the T. spiralis (0.02788 vs. 0.05862 μg/ml) and the N. brasiliensis (0.06188 vs. 0.1485 μg/ml) motility assays but not in the acetylcholine esterase secretion assay with adult N. brasiliensis (0.05650 vs. 0.06886 μg/ml). While amidantel showed only minimal or at best partial inhibition of nematode motility and acetylcholine esterase secretion, tribendimidine was nearly as potent as deacylated amidantel. Whereas deacylated amidantel had a significantly lower EC50 than tribendimidine in the N. brasiliensis L3 motility assay (0.05492 vs. 0.2080 μg/ml), differences were not significant in the T. spiralis L1 motility assay (0.7766 vs. 1.145 μg/ml). Surprisingly, none of the combinations showed improved efficacy when compared to the individual drugs including levamisole/tribendimidine, which have previously been reported to act synergistically against Ancylostoma ceylanicum.



Similar content being viewed by others
References
Conder GA, Johnson SS, Nowakowski DS, Blake TE, Dutton FE, Nelson SJ, Thomas EM, Davis JP, Thompson DP (1995) Anthelmintic profile of the cyclodepsipeptide PF1022A in in vitro and in vivo models. J Antibiot 48(8):820–823
Crompton DWT, World Health Organization (2006) Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. World Health Organization, Geneva
Crompton DWT, Savioli L (2007) Handbook of helminthiasis for public health. CRC/Taylor & Francis, Boca Raton
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Geary TG, Mackenzie CD (2011) Progress and challenges in the discovery of macrofilaricidal drugs. Expert Rev Anti Infect Ther 9(8):681–695
Guest M, Bull K, Walker RJ, Amliwala K, O’Connor V, Harder A, Holden-Dye L, Hopper NA (2007) The calcium-activated potassium channel, SLO-1, is required for the action of the novel cyclo-octadepsipeptide anthelmintic, emodepside, in Caenorhabditis elegans. Int J Parasitol 37(14):1577–1588
Gulick RM et al (1997) Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med 337(11):734–739
Harder A, von Samson-Himmelstjerna G (2002) Cyclooctadepsipeptides—a new class of anthelmintically active compounds. Parasitol Res 88(6):481–488
Harder A, Schmitt-Wrede HP, Krucken J, Marinovski P, Wunderlich F, Willson J, Amliwala K, Holden-Dye L, Walker R (2003) Cyclooctadepsipeptides—an anthelmintically active class of compounds exhibiting a novel mode of action. Int J Antimicrob Ag 22(3):318–331
Hu Y, Xiao SH, Aroian RV (2009) The new anthelmintic tribendimidine is an L-type (levamisole and pyrantel) nicotinic acetylcholine receptor agonist. PLoS Negl Trop Dis 3(8):e499
Knopp S et al (2010) Albendazole and mebendazole administered alone or in combination with ivermectin against Trichuris trichiura: a randomized controlled trial. Clin Infect Dis 51(12):1420–1428
Kotze AC, Lowe A, O’Grady J, Kopp SR, Behnke JM (2009) Dose–response assay templates for in vitro assessment of resistance to benzimidazole and nicotinic acetylcholine receptor agonist drugs in human hookworms. AmJTrop Med Hyg 81(1):163–170
Krücken J, Harder A, Jeschke P, Holden-Dye L, O’Connor V, Welz C, von Samson-Himmelstjerna G (2012) Anthelmintic cyclcooctadepsipeptides: complex in structure and mode of action. Trends Parasitol 28:385–394. doi:10.1016/j.pt.2012.06.005
Kulke D, Krucken J, Welz C, von Samson-Himmelstjerna G, Harder A (2012) In vivo efficacy of the anthelmintic tribendimidine against the cestode Hymenolepis microstoma in a controlled laboratory trial. Acta Trop 123(2):78–84
Leathwick DM, Hosking BC, Bisset SA, McKay CH (2009) Managing anthelmintic resistance: is it feasible in New Zealand to delay the emergence of resistance to a new anthelmintic class? New Zeal Vet J 57(4):181–192
Li RH, Gao JH, Wang SF, Pei YJ, Shen JY, Yan PM, Yin GR (2011) Effect of oral administration of tribendimidine at different dosages against Trichinella spiralis encapsulated larvae in mice. Chin J Parasitol Parasit Dis 29(2):117–121
Lumley AM, Lee DL (1981) Nippostrongylus brasiliensis and Nematodirus battus: changes in numbers and weight during the course of infection. Exp Parasitol 52(2):183–190
Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, Basanez MG (2012) A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl Trop Dis 6(4):e1582
Martin RJ, Robertson AP, Buxton SK, Beech RN, Charvet CL, Neveu C (2012) Levamisole receptors: a second awakening. Trends Parasitol 28(7):289–296
Mehlhorn H, Schmahl G, Frese M, Mevissen I, Harder A, Krieger K (2005) Effects of a combinations of emodepside and praziquantel on parasites of reptiles and rodents. Parasitol Res 97(1):65–69
Nicolay F, Harder A, von Samson-Himmelstjerna G, Mehlhorn H (2000) Synergistic action of a cyclic depsipeptide and piperazine on nematodes. Parasitol Res 86(12):982–992
Ogilvie BM, Rothwell TL, Bremner KC, Schnitzerling HJ, Nolan J, Keith RK (1973) Acetylcholinesterase secretion by parasitic nematodes. I. Evidence for secretion of the enzyme by a number of species. Int J Parasitol 3(5):589–597
Olliaro P, Seiler J, Kuesel A, Horton J, Clark JN, Don R, Keiser J (2011) Potential drug development candidates for human soil-transmitted helminthiases. PLoS Negl Trop Dis 5(6):e1138
Olsen A (2007) Efficacy and safety of drug combinations in the treatment of schistosomiasis, soil-transmitted helminthiasis, lymphatic filariasis and onchocerciasis. T Roy Soc Trop Med H 101(8):747–758
Prichard RK, Basanez MG, Boatin BA, McCarthy JS, Garcia HH, Yang GJ, Sripa B, Lustigman S (2012) A research agenda for helminth diseases of humans: intervention for control and elimination. PLoS Negl Trop Dis 6(4):e1549
Rapson EB, Lee DL, Watts SD (1981) Changes in the acetylcholinesterase activity of the nematode Nippostrongylus brasiliensis following treatment with benzimidazoles in vivo. Mol Biochem Parasitol 4(1–2):9–15
Rothwell TL, Ogilvie BM, Love RJ (1973) Acetylcholinesterase secretion by parasitic nematodes. II. Trichostrongylus spp. Int J Parasitol 3(5):599–608
Speich B, Ame SM, Ali SM, Alles R, Hattendorf J, Utzinger J, Albonico M, Keiser J (2012) Efficacy and safety of nitazoxanide, albendazole, and nitazoxanide-albendazole against Trichuris trichiura infection: a randomized controlled trial. PLoS Negl Trop Dis 6(6):e1685
Steinmann P, Zhou XN, Du ZW, Jiang JY, Xiao SH, Wu ZX, Zhou H, Utzinger J (2008) Tribendimidine and albendazole for treating soil-transmitted helminths, Strongyloides stercoralis and Taenia spp.: open-label randomized trial. PLoS Negl Trop Dis 2(10):e322
Tomlinson G, Albuquerque CA, Woods RA (1985) The effects of amidantel (BAY d 8815) and its deacylated derivative (BAY d 9216) on Caenorhabditis elegans. Eur J Pharmacol 113(2):255–262
Tritten L, Nwosu U, Vargas M, Keiser J (2012) In vitro and in vivo efficacy of tribendimidine and its metabolites alone and in combination against the hookworms Heligmosomoides bakeri and Ancylostoma ceylanicum. Acta Trop 122(1):101–107
von Samson-Himmelstjerna G, Harder A, Schnieder T, Kalbe J, Mencke N (2000) In vivo activities of the new anthelmintic depsipeptide PF 1022A. Parasitol Res 86(3):194–199
Wang M, Watanabe N, Shomura T, Ohtomo H (1994) Effects of PF1022A from Mycelia sterilia on Trichinella spiralis in mice. Jpn J Parasitol 43(5):346–350
Wang M, Watanabe N, Shomura T, Ohtomo H (1995) Effects of PF1022A on Nippostrongylus brasiliensis in rats and Hymenolepis nana in mice. Jpn J Parasitol 44(4):306–310
Watts SD, Rapson EB, Atkins AM, Lee DL (1982) Inhibition of acetylcholinesterase secretion from Nippostrongylus brasiliensis by benzimidazole anthelmintics. Biochem Pharmacol 31(19):3035–3040
Weaver HJ, Hawdon JM, Hoberg EP (2010) Soil-transmitted helminthiases: implications of climate change and human behavior. Trends Parasitol 26(12):574–581
Welz C, Kruger N, Schniederjans M, Miltsch SM, Krucken J, Guest M, Holden-Dye L, Harder A, von Samson-Himmelstjerna G (2011) SLO-1-channels of parasitic nematodes reconstitute locomotor behaviour and emodepside sensitivity in Caenorhabditis elegans slo-1 loss of function mutants. PLoS Pathog 7(4):e1001330
Wollweber H, Niemers E, Flucke W, Andrews P, Schulz HP, Thomas H (1979) Amidantel, a potent anthelminthic from a new chemical class. Arzneim Forsch 29(1):31–32
Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC (2004) Drug resistance in veterinary helminths. Trends Parasitol 20(10):469–476
Woods RA, Malone KMB (1985) The effects of amidantel (BAY d 8815) and its deacylated derivative (BAY d 9216) on wild-type and resistant mutants of Caenorhabditis elegans. Can J Zool 64:1310–1316
World Health Organization (2011a) WHO model list of essential medicines: 17th list, March 2011. World Health Organization, Geneva
World Health Organization (2011b) WHO model list of essential medicines for children: 3rd list, March 2011. World Health Organization, Geneva
Xiao SH, Hui-Ming W, Tanner M, Utzinger J, Chong W (2005) Tribendimidine: a promising, safe and broad-spectrum anthelmintic agent from China. Acta Trop 94(1):1–14
Xue J, Xiao SH, Xu LL, Qiang HQ (2010a) The effect of tribendimidine and its metabolites against Necator americanus in golden hamsters and Nippostrongylus braziliensis in rats. Parasitol Res 106(4):775–781
Xue J, Xiao SH, Xu LL, Zhang YN, Qiang HQ (2010b) Efficacy of tribendimidine and albendazole in treating mice infected with Trichinella spiralis. Chin J Parasitol Parasit Dis 28(1):8–11
Yuan G, Xu J, Qu T, Wang B, Zhang R, Wei C, Guo R (2010) Metabolism and disposition of tribendimidine and its metabolites in healthy Chinese volunteers. Drugs R D 10(2):83–90
Acknowledgements
The Bayer HealthCare AG (Leverkusen, Germany) funded the presented study. The Bayer HealthCare AG sells anthelmintics for the use in veterinary medicine.
Conflict of interest
A. Harder and D. Kulke were employees of Bayer HealthCare AG when the study was conducted. Bayer HealthCare had no influence on the design of the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kulke, D., Krücken, J., Demeler, J. et al. In vitro efficacy of cyclooctadepsipepdtides and aminophenylamidines alone and in combination against third-stage larvae and adult worms of Nippostrongylus brasiliensis and first-stage larvae of Trichinella spiralis . Parasitol Res 112, 335–345 (2013). https://doi.org/10.1007/s00436-012-3141-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-3141-1