Abstract
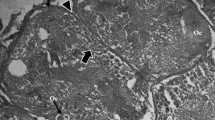
The formation and uptake of the yolk protein in the oocyte of the Asian Tiger, Aedes albopictus mosquito was investigated. Light and electron microscopy of the ovaries at early resting stage as well as the structural changes associated with yolk formation were described 16 h after blood meal. The deposition of the yolk protein in the oocyte was correlated with a 15-fold increase in 138-μm pit-like depressions on the oocyte surface. These pits result by invagination of the oocyte cell membrane. They have a 20-μm bristle coat on their convex cytoplasmic side and a layer of protein on their concave extraoocyte space. The pits, by pinching off from the cell membrane become bristle coat vesicles which carry the adsorbed protein into the oocyte. These vesicles lose the coat and then fuse to form small crystalline yolk droplets, which subsequently coalesce to form the large protein yolk bodies of the mature oocyte. Preliminary radioautographs and certain morphological features of the fat body, ovary, and midgut, suggest that the midgut is the principal site of the yolk protein synthesis in A. albopictus.











Similar content being viewed by others
References
Anderson E (1962) The ovary of Periplaneta americana, Abstracts of the 2nd Annual Meeting of the American Society for Cell Biology, San Francisco, 2
Ashford TP, Porter KR (1962) Cytoplasmic components in hepatic cell lysosomes. J Cell Biol 12:198–202
Beams HW, Kessel RG (1962) Endoplasmic reticulum and yolk formation in crayfish, Abstracts of the 2nd Annual Meeting of the American Society for Cell Biology, San Francisco, 13
Brambell FWR, Hemmings WA (1954) Active transport through embryonic membranes. Syrup Soc Exp Biol 8:476
Brandt PW (1958) A study of the mechanism of pinocytosis. Exp Cell Research 15:300
Chargaff E (1942) The formation of the phosphorus compounds in egg yolk. J Biol Chem 142:505
Farquhar MG, Palade GE (1961) Glomerular capillary wall in nephritic rats. J Exp Med 114:699
Favard P, Carasso N (1958) Origin et ultrastructure des plaquettes vitellines de la Planorbe. Arch Anat Micro et Morph Exp 47:211
Flickinger RA (1960) Formation, biochemical composition and utilization of amphibian egg yolk, in Symposium on Germ Cells and Development, Institut Internationale d’Embryologie and Fondazione A. Baselli, Pavia, Italy, Premiata Tipografia Successore Fratelli Fusi, 29
Grant P (1953) Phosphate metabolism during oogenesis in Rana temporaria. J Exp Zool 124:513
Hahn L, Hevesy G (1937) Origin of yolk lecithin. Nature 140:1059
Hosoda T, Kaneko Z, Mogl K, Abe T (1955) Serological studies on egg production in the fowl. I. On the locus of serum vitellin production. Poultry Sci 34:9
Jukes TH, Kay HD (1932) Egg yolk protein. J Nutrition 5:81
King RC (1960) Oogenesis in adult Drosophila melanogaster. IX. Studies on the cytochemistry and ultrastructure of developing oocytes. Growth 24:265
Knight PF, Schechtman AM (1954) The passage of heterologons serum proteins from the circulation into the ovum of the fowl. J Exp Zool 127:271
Luft JH (1961) Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol 9:409–410
Mancini RE, Vilar O, Heinrich JJ, Davidson OW, Alvarez B (1963) Transference of circulating labeled serum proteins to the follicle of the rat ovary. J Histochem Cytochem 11:80
Miller F (1960) Hemoglobin absorption by the cells of the proximal convoluted tubule in mouse kidney. J Biophysic Biochem Cytol 8:689
Nath VM (1924) Egg-follicle of Culex. Q J Microsc Sci 65:152–175
Nath V (1929) Studies on the shape of the Golgi apparatus. I. The egg follicle of Culex. Z Zellforsch 8:655
Nicholson AJ (1920) The development of the ovary and ovarian egg of a mosquito, Anopheles maculipennis. Quart J Micr Sc 65:395
Palade GE (1960) Transport in quanta across the endothelium of blood capillaries. Anat Rec 136:254
Roepke RR, Bushnell LD (1936) A serological comparison of the phosphoproteins of the serum of the laying hen and the vitellin of the egg yolk. J Immunol 30:109
Roth TF, Porter KR (1962) Specialized sites on the cell surface for protein uptake. In Breese SS Jr. (eds), 5th International Congress for Electron Microscopy, Philadelphia, New York, Academic Press, Inc., 2, LL-4
Roth TF, Porter KR (1963) Membrane differentiation for protein uptake. Fed Proc 22(No. 2)
Roth TF, Porter KR (1964) Yolk protein uptake in the oocyte of the mosquito Aedes aegypti L. J Cell Biology 20:313–332
Schjeide OA, Urist MR (1959) Proteins and calcium in serums of estrogen treated roosters. Science 124:1242
Smith AH (1959) Follicular permeability and yolk formation. Poultry Sci 38:1437
Straus W (1962) Cytochemieal investigation of phagosomes and related structures in cryostat sections of the kidney and liver of rats after intravenous administration of horseradish peroxidase. Exp Cell Research 27:80
Telfer WH (1954) Immunological studies of insect metamorphosis. II. The role of a sex-limited blood protein in egg formation by the Cecropia silkworm. J Gen Physiol 37:539
Telfer WH (1960) The selective accumulation of blood proteins by the oocytes of saturniid moths. Biol Bull 118:338
Telfer WH (1961) The route of entry and localization of blood proteins in the oocytes of saturniid moths. J Biophysic Biochem Cytol 9:747
Ward RT (1962) The origin of protein and fatty yolk in Rana pipiens. II. Electron microscopical and cytochemical observations on young and mature oocytes. J Cell Biol 14:309
Warttenberg H (1962) Electronen mikroskopische und Histochemische Studien uber die Oogenese der Amphibieneizelle Z. Zellforsch 58:427
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamany, A.S., Mehlhorn, H. & Adham, F.K. Yolk protein uptake in the oocyte of the Asian tiger mosquito Aedes albopictus (Skuse) (Diptera: Culicidae). Parasitol Res 111, 1315–1324 (2012). https://doi.org/10.1007/s00436-012-2967-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-2967-x