Abstract
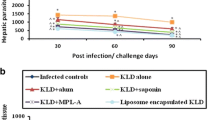
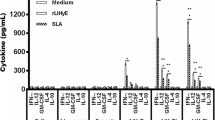
Development of an effective vaccine against leishmaniasis is possible due to the fact that individuals cured from cutaneous leishmaniasis (CL) are protected from further infection. First generation Leishmania vaccines consisting of whole killed parasites reached to phase 3 clinical trials but failed to show enough efficacies mainly due to the lack of an appropriate adjuvant. In this study, an efficient liposomal protein-based vaccine against Leishmania major infection was developed using soluble Leishmania antigens (SLA) as a first generation vaccine and cytidine phosphate guanosine oligodeoxynucleotides (CpG ODNs) as an immunostimulatory adjuvant. 1, 2-Dioleoyl-3-trimethylammonium-propane was used as a cationic lipid to prepare the liposomes due to its intrinsic adjuvanticity. BALB/c mice were immunized subcutaneously (SC), three times in 2-week intervals, with Lip-SLA-CpG, Lip-SLA, SLA + CpG, SLA, or HEPES buffer. As criteria for protection, footpad swelling at the site of challenge and spleen parasite loads were assessed, and the immune responses were evaluated by determination of IFN-γ and IL-4 levels of cultured splenocytes, and IgG subtypes. The group of mice that received Lip-SLA-CpG showed a significantly smaller footpad swelling, lower spleen parasite burden, higher IgG2a antibody, and lower IL-4 level compared to the control groups. It is concluded that cationic liposomes containing SLA and CpG ODNs are appropriate to induce Th1 type of immune response and protection against leishmaniasis.






Similar content being viewed by others
References
Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P (1994) The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science 263(5144):235–237
Afrin F, Rajesh R, Anam K, Gopinath M, Pal S, Ali N (2002) Characterization of Leishmania donovani antigens encapsulated in liposomes that induce protective immunity in BALB/c mice. Infect Immun 70(12):6697–6706
Badiee A, Jaafari MR, Khamesipour A (2007) Leishmania major: immune response in BALB/c mice immunized with stress-inducible protein 1 encapsulated in liposomes. Exp Parasitol 115(2):127–134
Badiee A, Jaafari MR, Samiei A, Soroush D, Khamesipour A (2008) Coencapsulation of CpG oligodeoxynucleotides with recombinant Leishmania major stress-inducible protein 1 in liposome enhances immune response and protection against leishmaniasis in immunized BALB/c mice. Clin Vaccine Immunol 15(4):668–674
Bhowmick S, Ravindran R, Ali N (2007) Leishmanial antigens in liposomes promote protective immunity and provide immunotherapy against visceral leishmaniasis via polarized Th1 response. Vaccine 25(35):6544–6556
Chen W, Yan W, Huang L (2008) A simple but effective cancer vaccine consisting of an antigen and a cationic lipid. Cancer Immunol Immun 57(4):517–530
Chikh GG, Kong S, Bally MB, Meunier JC, Schutze-Redelmeier MP (2001) Efficient delivery of Antennapedia homeodomain fused to CTL epitope with liposomes into dendritic cells results in the activation of CD8+ T cells. J Immunol 167(11):6462–6470
Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV (1997) CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med 186(10):1623–1631
Coler RN, Reed SG (2005) Second-generation vaccines against leishmaniasis. Trends Parasitol 21(5):244–249
Croft SL, Sundar S, Fairlamb AH (2006) Drug resistance in leishmaniasis. Clin Microbiol Rev 19(1):111–126
De Oliveira MC, Boutet V, Fattal E, Boquet D, Grognet J-M, Couvreur P, Deverre J-R (2000) Improvement of in vivo stability of phosphodiester oligonucleotide using anionic liposomes in mice. Life Sci 67(13):1625–1637
Dumont FJ (2002) Modulation of Th1 and Th2 responses for immunotherapy. Expert Opin Ther Pat 12(3):341–367
Firooz A, Khamesipour A, Ghoorchi MH, Nassiri-Kashani M, Eskandari SE, Khatami A, Hooshmand B, Gorouhi F, Rashighi-Firoozabadi M, Dowlati Y (2006) Imiquimod in combination with meglumine antimoniate for cutaneous leishmaniasis: a randomized assessor-blind controlled trial. Arch Dermatol 142(12):1575–1579
Hafez IM, Maurer N, Cullis PR (2001) On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther 8(15):1188–1196
Handman E (2001) Leishmaniasis: current status of vaccine development. Clin Microbiol Rev 14(2):229–243
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408(6813):740–745
Hoekstra D, Rejman J, Wasungu L, Shi F, Zuhorn I (2007) Gene delivery by cationic lipids: in and out of an endosome. Biochem Soc T 035(1):68–71
Ignatius R, Mahnke K, Rivera M, Hong K, Isdell F, Steinman RM, Pope M, Stamatatos L (2000) Presentation of proteins encapsulated in sterically stabilized liposomes by dendritic cells initiates CD8+ T-cell responses in vivo. Blood 96(10):3505–3513
Jaafari MR, Badiee A, Khamesipour A, Samiei A, Soroush D, Kheiri MT, Barkhordari F, McMaster WR, Mahboudi F (2007) The role of CpG ODN in enhancement of immune response and protection in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63) encapsulated in cationic liposome. Vaccine 25(32):6107–6117
Jiao X, Wang RY-H, Qiu Q, Alter HJ, Shih JW-K (2004) Enhanced hepatitis C virus NS3 specific Th1 immune responses induced by co-delivery of protein antigen and CpG with cationic liposomes. J Gen Virol 85(6):1545–1553
Karmali PP, Chaudhuri A (2007) Cationic liposomes as non-viral carriers of gene medicines: resolved issues, open questions, and future promises. Med Res Rev 27(5):696–722
Keshavarz Valian H, Khoshabe Abdollah Kenedy L, Nateghi Rostami M, Miramin Mohammadi A, Khamesipour A (2008) Role of Mycobacterium vaccae in the protection induced by first generation Leishmania vaccine against murine model of leishmaniasis. Parasitol Res 103(1):21–28
Khalil EA, El Hassan AM, Zijlstra EE, Mukhtar MM, Ghalib HW, Musa B, Ibrahim ME, Kamil AA, Elsheikh M, Babiker A, Modabber F (2000) Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-blind, BCG-controlled trial in Sudan. Lancet 356(9241):1565–1569
Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F (2006) Leishmaniasis vaccine candidates for development: a global overview. Indian J Med Res 123(3):423–438
Klinman DM (2004) Use of CpG oligodeoxynucleotides as immunoprotective agents. Expert Opin Biol Ther 4(6):937–946
Klinman DM, Currie D, Gursel I, Verthelyi D (2004) Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev 199(1):201–216
Krieg AM (2000) The role of CpG motifs in innate immunity. Curr Opin Immunol 12(1):35–43
Krieg AM (2002) CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20(1):709–760
Levin AA (1999) A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides. Biochim Biophys Acta 1489(1):69–84
Li WM, Dragowska WH, Bally MB, Schutze-Redelmeier M-P (2003) Effective induction of CD8+ T-cell response using CpG oligodeoxynucleotides and HER-2/neu-derived peptide co-encapsulated in liposomes. Vaccine 21(23):3319–3329
Manuel S, Ramirez L, Pineda MA, Gonzalez V, Entringer PF, de Oliveira CI, Nascimento IP, Souza AP, Corvo L, Alonso C, Bonay P, Brodskyn C, Barral A, Barral-Netto M, Iborra S (2009) Searching genes encoding Leishmania antigens for diagnosis and protection. Scholarly Res Exchange 2009
Marques-da-Silva EA, Coelho EA, Gomes DC, Vilela MC, Masioli CZ, Tavares CA, Fernandes AP, Afonso LC, Rezende SA (2005) Intramuscular immunization with p36(LACK) DNA vaccine induces IFN-gamma production but does not protect BALB/c mice against Leishmania chagasi intravenous challenge. Parasitol Res 98(1):67–74
Mazumder S, Ravindran R, Banerjee A, Ali N (2007) Non-coding pDNA bearing immunostimulatory sequences co-entrapped with leishmanial antigens in cationic liposomes elicits almost complete protection against experimental visceral leishmaniasis in BALB/c mice. Vaccine 25(52):8771–8781
Melby PC (2002) Vaccination against cutaneous leishmaniasis: current status. Am J Clin Dermatol 3(8):557–570
Modabber F (1995) Vaccines against leishmaniasis. Ann Trop Med Parasitol 89(Suppl 1):83–88
Momeni AZ, Jalayer T, Emamjomeh M, Khamesipour A, Zicker F, Ghassemi RL, Dowlati Y, Sharifi I, Aminjavaheri M, Shafiei A, Alimohammadian MH, Hashemi-Fesharki R, Nasseri K, Godal T, Smith PG, Modabber F (1999) A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine 17(5):466–472
Musa AM, Khalil EA, Mahgoub FA, Elgawi SH, Modabber F, Elkadaru AE, Aboud MH, Noazin S, Ghalib HW, El-Hassan AM (2008) Immunochemotherapy of persistent post-kala-azar dermal leishmaniasis: a novel approach to treatment. Trans R Soc Trop Med Hyg 102(1):58–63
Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, Fujiwara H, Hamaoka T, Mayumi T (1997) Positively charged liposome functions as an efficient immunoadjuvant in inducing immune responses to soluble proteins. Biochem Biophys Res Co 240(3):793–797
Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, Nakanishi M, Tanaka K, Mayumi T (1999) Positively charged liposome functions as an efficient immunoadjuvant in inducing cell-mediated immune response to soluble proteins. J Control Release 61(1–2):233–240
Noazin S, Modabber F, Khamesipour A, Smith PG, Moulton LH, Nasseri K, Sharifi I, Khalil EA, Bernal ID, Antunes CM, Kieny MP, Tanner M (2008) First generation leishmaniasis vaccines: a review of field efficacy trials. Vaccine 26(52):6759–6767
Noazin S, Khamesipour A, Moulton LH, Tanner M, Nasseri K, Modabber F, Sharifi I, Khalil EAG, Bernal IDV, Antunes CMF, Smith PG (2009) Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis—a meta-analysis. Vaccine 27(35):4747–4753
Rao M, Alving CR (2000) Delivery of lipids and liposomal proteins to the cytoplasm and Golgi of antigen-presenting cells. Adv Drug Deliver Rev 41(2):171–188
Rhee EG, Mendez S, Shah JA, C-Y Wu, Kirman JR, Turon TN, Davey DF, Davis H, Klinman DM, Coler RN, Sacks DL, Seder RA (2002) Vaccination with heat-killed Leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J Exp Med 195(12):1565–1573
Santos DO, Coutinho CE, Madeira MF, Bottino CG, Vieira RT, Nascimento SB, Bernardino A, Bourguignon SC, Corte-Real S, Pinho RT, Rodrigues CR, Castro HC (2008) Leishmaniasis treatment—a challenge that remains: a review. Parasitol Res 103(1):1–10
Scott P, Pearce E, Natovitz P, Sher A (1987) Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol 139(1):221–227
Sharifi I, FeKri AR, Aflatonian MR, Khamesipour A, Nadim A, Mousavi MR, Momeni AZ, Dowlati Y, Godal T, Zicker F, Smith PG, Modabber F (1998) Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet 351(9115):1540–1543
Suzuki Y, Wakita D, Chamoto K, Narita Y, Tsuji T, Takeshima T, Gyobu H, Kawarada Y, Kondo S, Akira S, Katoh H, Ikeda H, Nishimura T (2004) Liposome-encapsulated CpG oligodeoxynucleotides as a potent adjuvant for inducing type 1 innate immunity. Cancer Res 64(23):8754–8760
Tafaghodi M, Khamesipour A, Jaafari MR (2010) Immunization against leishmaniasis by PLGA nanospheres encapsulated with autoclaved Leishmania major (ALM) and CpG-ODN. Parasitol Res 108(5):1265–1273
Taswell C (1981) Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol 126(4):1614–1619
Vangasseri DP, Cui Z, Chen W, Hokey DA, Falo LD, Huang L (2006) Immunostimulation of dendritic cells by cationic liposomes. Mol Membr Biol 23(5):385–395
Wasungu L, Hoekstra D (2006) Cationic lipids, lipoplexes and intracellular delivery of genes. J Control Release 116(2):255–264
Weeratna RD, Brazolot Millan CL, McCluskie MJ, Davis HL (2001) CpG ODN can re-direct the Th bias of established Th2 immune responses in adult and young mice. FEMS Immunol Med Microbiol 32(1):65–71
Wilson KD, de Jong SD, Tam YK (2009) Lipid-based delivery of CpG oligonucleotides enhances immunotherapeutic efficacy. Adv Drug Deliver Rev 61(3):233–242
Yan W, Chen W, Huang L (2007) Mechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokines. Mol Immunol 44(15):3672–3681
Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ (1995) Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem 270(32):18997–19007
Zhang YM, Rusckowski M, Liu N, Liu C, Hnatowich DJ (2001) Cationic liposomes enhance cellular/nuclear localization of 99mTc-antisense oligonucleotides in target tumor cells. Cancer Biother Radiopharm 16(5):411–419
Acknowledgments
The financial support of the Nanotechnology Research Center and Biotechnology Research Center, Mashhad University of Medical Sciences (MUMS), and Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences (TUMS) are gratefully acknowledged. This study was part of Pharm. D. dissertation of VHS that was done in Nanotechnology Research Center, MUMS, Iran.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ali Badiee and Mahmoud Reza Jaafari equally designed this research.
Rights and permissions
About this article
Cite this article
Heravi Shargh, V., Jaafari, M.R., Khamesipour, A. et al. Cationic liposomes containing soluble Leishmania antigens (SLA) plus CpG ODNs induce protection against murine model of leishmaniasis. Parasitol Res 111, 105–114 (2012). https://doi.org/10.1007/s00436-011-2806-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2806-5