Abstract
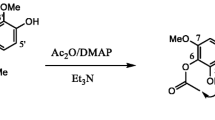
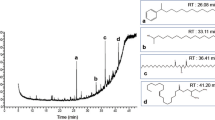
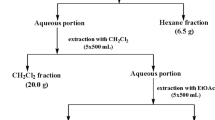
Extracts (34) from eight plant species of the Peruvian Amazonia currently used in traditional Peruvian medicine, mostly as antileishmanial remedies and also as painkiller, antiseptic, antipyretic, anti-inflamatory, antiflu, astringent, diuretic, antipoison, anticancerous, antiparasitic, insecticidal, or healing agents, have been tested for their antileishmanial, antitrypanosomal, and cytotoxic activity. Plant species were selected based on interviews conducted with residents of rural areas. The different plant parts were dried, powdered, and extracted by maceration with different solvents (hexane, chloroform, and 70% ethanol–water). These extracts were tested on promastigote forms of Leishmania infantum strain PB75, epimastigote forms of Trypanosoma cruzi strain Y, and the mammalian CHO cell line. Parasite viability and nonspecific cytotoxicity were analyzed by a modified MTT colorimetric assay method. The isolation and identification of pure compounds from selected extracts were performed by column chromatography, gas chromatography mass spectrometry (GC-MS; mixtures), spectroscopic techniques [MS, infrared (IR), ultraviolet (UV)], and mono and two-dimensional 1H and 13C nuclear magnetic resonance (NMR; COSY, HSQC, NOESY) experiments. Chondodendron tomentosum bark and Cedrela odorata were the most active extracts against Leishmania, while C. odorata and Aristoloquia pilosa were the most active against Trypanosoma, followed by Tabebuia serratifolia, Tradescantia zebrina, and Zamia ulei. Six compounds and two mixtures were isolated from Z. ulei [cycasin (1)], T. serratifolia {mixtures 1–2, and naphthoquinones 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione (2) and 2-(1-hydroxyethyl)-4H,9H-naphtho[2,3-b]furan-4,9-dione (3)}, and C. tomentosum [chondrocurine (4); (S,S′)-12-O-methyl(+)-curine (5); and cycleanine (6)]. Four compounds and the two mixtures exhibited significant activity.


Similar content being viewed by others
References
Angerhofer C, Guinaudeau H, Wongpanich V, Pezzuto JM, Cordell A (1999) Antiplasmodial and cytotoxic activity of natural bisbenzylisoquinoline alkaloids. J Nat Prod 62:59–66
Cao S, Schilling JK, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DG (2004) Cytotoxic compounds from Mundulea chapelieri from the Madagascar Rainforest. J Nat Prod 67:454–456
Colman de Saizarbitoria T, Anderson JE, Alfonso D, MacLaughlin JL (1997) Bioactive furonaphtoquinones from Tabebuia barbata, Bignoniaceae. Acta Cient Venez 48:42–46
Correa DS, Tempone AG, Reimao JQ, Taniwaki NN, Romoff P, Fávero OA, Sartorelli P, Mecchi MC, Lago JHG (2011) Anti-leishmanial and anti-trypanosomal potential of polygodial isolated from stem barks of Drimys brasiliensis. Parasitol Res 109:231–236
Croft SL, Barrett MP, Urbina JA (2005) Chemotherapy of trypanosomiases and leishmaniasis. Trends Parasitol 21:508–512
Do Campo R, Lopes JN, Cruz FS, De Soua W (1977) Trypanosoma cruzi: ultrastructural and metabolic alterations of epimastigotes by β-lapachone. Exp Parasitol 42:142–149
Do Campo R, Cruz FS, Boveris A, Muniz RP, Esquibel DM (1978) Lipid peroxidation and the generation of free radicals, superoxide anion, and hydrogen peroxide in beta-lapachone-treated Trypanosoma cruzi epimastigotes. Arch Biochem Biophys 186:292–297
Everette AJ, Lowe LA, Wilkinson S (1970) Revision of the structure of (+)-tubocurarine chloride and (+)-chondrocurine. J Chem Soc D 1970:1020–1021
Estevez Y, Castillo D, Pisango MT, Arevalo J, Rojas R, Alban J, Deharo E, Bourdy G, Sauvain M (2007) Evaluation of the leishmanicidal activity of plants used by Peruvian Chayahuita ethnic group. J Ethnopharmacol 114:254–259
Fournet A, Muñoz V (2002) Natural products as trypanocidal, antileishmanial and antimalarial drugs. Curr Top Med Chem 2:1215–1237
Fournet A, Ferreira ME, Rojas de Arias A, Schinini A, Nakayama H, Torres S, Sanabria R, Guinaudeau H, Bruneton J (1997) The effects of bisbenzylisoquinoline alkaloids in T. cruzi infections in mice. Int J Antimicrob Agents 8:163–170
Fournet A, Inchaustti A, Yaluff G, Rojas de Arias A, Guinaudeau H, Bruneton J, Breidenbach MA, Karplus PA, Faerman CH (1998) Trypanocidal bisbenzylisoquinoline alkaloids are inhibitors of trypanothione reductasa. J Enzyme Inhib 13:1–9
Gascon J, Bern C, Pinazo MJ (2010) Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 115:22–27
González-Coloma A, Guadaño A, de Inés C, Martínez-Díaz R, Cortés D (2002) Selective action of acetogenin mitochondrial Complex I inhibitors. Z Naturforsch 57C:1028–1034
Ghosh S, Debnath S, Hazra S, Hartung A, Thomale K, Schutheis M, Kapkova P, Schurigt U, Moll H, Holzgrabe U, Hazra B (2011) Valeriana wallichii root extracts and fractions with activity against Leishmania spp. Parasitol Res 108:861–871
Haynes LJ, Herbert EJ, Plimmer JR (1966) (+ +)-4″-O-Methylcurine from Cissampelos pareira. J Chem Soc C 1966:615–617
Hussain H, Krohn K, Uddin Ahmad V, Abbas Miana G, Robert Green I (2007) Lapachol: an overview. ARKIVOC 2:145–171
Karabay NU, Sukatar A, Ozdemir G, Horzum Z (2007) Antimicrobial activity of volatile components and various extracts of the red alga Jania rubens. Phytother Res 21:153–156
Kuete V, Eyong KO, Folefoc GN, Beng VP, Hussain H, Krohn K, Nkengfack AE (2007) Antimicrobial activity of the methanolic extract and the chemical constituents isolated from Newbouilda laevis. Die Pharmazie 62:552–556
Kuiate JR, Bessiere JM, Zollo PHA, Kuate SP (2006) Chemical composition and antidermatophytic properties of volatile fractions of hexanic extract from leaves of Cupressus lusitanica Mill. from Cameroon. J Ethnopharmacol 103:160–165
Kvist LP, Christensen SB, Rasmussen HB, Mejía K, González A (2006) Identification and evaluation of Peruvian plants used to treat malaria and leishmaniasis. J Ethnopharmacol 106:390–402
Leboeuf M, Cavé A, Bhaunik PK, Mukherjee B, Mukherjee R (1982) The phytochemistry of the Annonaceae. Phytochemistry 21:2783–2813
Lin LZ, Shied HL, Angerhofer CK, Pezzuto JM, Cordell GA (1993) Citotoxic and antimalarial bisbenzylisoquinoline alkaloids from Cyclea barbata. J Nat Prod 56:22–29
Lohombo-Ekomba ML, Okusa PN, Penge O, Kabongo C, Iqbal M, Kasende OE (2004) Antibacterial, antifungal, antiplasmodial and cytotoxic activities of Albertisia villosa. J Ethnopharmacol 93:331–335
Mackinnon S, Durst T, Arnason JT (1997) Antimalarial activity of tropical Meliaceae extracts and gedunin derivatives. J Nat Prod 60:336–341
Mazutti M, Mossi AJ, Cansian RL, Corazza ML, Dariva C, Oliveira V (2008) Chemical profile and antimicrobial activity of Boldo (Peumus boldus Molina). Extracts obtained by compressed carbon dioxide extraction. Braz J Chem Eng 25:427–434
Mejia K, Rengifo E (2000) Plantas medicinales de uso popular en la Amazonía Peruana. Instituto de Investigación de la Amazonía Peruana - Gobierno Regional de Loreto - Agencia Española de Cooperación Internacional, Lima
Mossman T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Muelas Serrano S, Nogal JJ, Martínez Díaz RA, Escario JA, Martínez Fernández AR, Gómez Barrio A (2000) In vitro screening of American plant extracts on Trypanosoma cruzi and Trichomonas vaginalis. J Ethnopharmacol 71:101–107
Muñoz J, Portús M, Corachan M, Fumadó V, Gascona J (2007) Congenital Trypanosoma cruzi infection in a non-endemic area. Trans R Soc Trop Med Hyg 101:1161–1162
Murray HW, Berman JD, Davies CR, Saravia NG (2005) Advances in leishmaniasis. Lancet 366:1561–1577
Nishida K, Kobayashi A, Nagahama T (1955) Studies on cycasin, a new toxic glycoside of Cycas revoluta Thunb. Part I. Isolation and structure of cycasin. Bull Agric Chem Soc Japan 19:77–83
Nishida K, Kobayashi A, Nagahama T (1956) Studies on cycasin, a new toxic glycoside of Cycas revoluta Thunb. Part III. Isolatión of cycasin from the stem of Japanese cycad. Seikagaku 28:70–74
Osorio J, Montoya G, Arango G (2006) Productos naturales alcaloidales con actividad antiprotozoaria. Vitae 13:61–84
Passero LFD, Bonfim-Melo A, Corbett CEP, Laurenti MD, Toyama MH, de Toyama DO, Romoff P, Fávero OA, dos Grecco SS, Zalewsky CA, Lago JHG (2011) Anti-leishmanial effects of purified compounds from aerial parts of Baccharis uncinella C. (Asteraceae). Parasitol Res 108:529–536
Perez D, Iannacone J (2006) Effectiveness of botanical extracts from ten plants on mortality and larval repellency of Rhynchophorus palmarum L., an insect pest of the Peach palm Bactris gasipaes Kunth in Amazonian Peru. Agric Tec 66:21–30
Rao MM, Kingston DGI (1982) Plant anticancer agents. XII. Isolation and structure elucidation of new cytotoxic quinones from Tabebuia cassinoides. J Nat Prod 45:600–604
Reina M, González-Coloma A, Gutiérrez C, Cabrera R, Rodríguez ML, Villarroel L, Fajardo V (2001) Defensive chemistry of Senecio miser Hook. J Nat Prod 64:6–11
Rojas R, Bustamante B, Bauer J, Fernández I, Albán J, Lock O (2003) Antimicrobial activity of selected Peruvian medicinal plants. J Ethnopharmacol 88:199–204
Sánchez-Cañete MP, Carvalho L, Pérez-Victoria FJ, Gamarro F, Castanys S (2009) Low plasma membrane expression of the miltefosine transport complex renders Leishmania braziliensis refractory to the drug. Antimicrob Agents Chemother 53:1305–1313
Schneider D, Wink M, Sporer F (2002) Cycads: their evolution, toxins, herbivores and insect pollinators. Naturwissenschaften 89:281–294
Scheinmann F, Scriven EFV, Ogbeibe ON (1980) Cycleanine from Synclisia scabrida: conformational information from the 1H NMR spectrum at 300 MHz. Phytochemistry 19:1837–1840
Sra KK, Sracic J, Tyring SK (2004) Treatment of protozoan infections. Dermatol Ther 17:513–516
Thornber CW (1970) Alkaloids of the Menispermaceae. Phytochemistry 9:157–187
Tshibangu JN, Wright AD, Köning GM (2003) HPLC isolation of the antiplasmodially active bisbenzylisoquinoline alkaloids present in roots of Cissampelos mucronata. Phytochem Anal 14:13–22
Weiss CR, Moideen SVK, Croft SL, Houghton PJ (2000) Activity of extracts and isolated naphthoquinones from Kigelia pinnata against Plasmodium falciparum. J Nat Prod 63:1306–1309
Yagi F (2004) Azoxyglycoside content and β-glycosidase activities in leaves of various cycads. Phytochemistry 65:3243–3247
Zai-Chang Y, Bo-Chu W, Xio-Sheng Y, Qiang W (2005) Chemical composition of the volatile oil from Cynanchum stauntonii and its activities of anti-influenza virus. Colloid Surf B Biointerfaces 43:198–202
Zani CL, De Oliveira AB, De Oliveira GG (1991) Furonaphthoquinones from Tabebuia ochracea. Phytochemistry 30:2379–2381
Acknowledgments
This work was supported by grant CTQ2009-14629-C02-01 (Spain) and a collaborative research project CSIC-CONCYTEC (Perú). We also thank S. Carlin for language revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Coloma, A., Reina, M., Sáenz, C. et al. Antileishmanial, antitrypanosomal, and cytotoxic screening of ethnopharmacologically selected Peruvian plants. Parasitol Res 110, 1381–1392 (2012). https://doi.org/10.1007/s00436-011-2638-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2638-3