Abstract
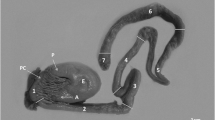
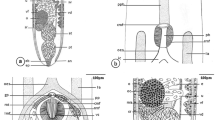
The results described the structure of Longicollum pagrosomi and histopathological characters of the intestine of the red sea bream, Pagrus major, infected with acanthocephalans, using the light and electron microscopes. Among the six samples of P. major, L. pagrosomi was identified in the posterior intestine of five fish samples. Adult L. pagrosomi (total length, 8–27 mm) is divided into the presoma (proboscis, anterior neck, and posterior neck) and metasoma (trunk). The proboscis had vertically arranged hooks (40 μm in length), with ten hooks per row, and the septum was observed between the posterior neck and trunk. The tegument thickness of the proboscis was approximately 15 μm, and it was composed of thin, circular muscle fibers. The outer fibrous membrane was approximately 1 μm, and the connective tissue layer was approximately 35 μm in thickness in the anterior neck. The tegument of the posterior neck enclosed the cephalic ganglion and had longitudinal and vertical muscle fibers, and the tegument thickness was approximately 45 μm. The tegument of the body, which was approximately 1 mm in thickness, was composed primarily of muscle and collagen fibers, and the structure of the tegument was different, depending on the body region. The acanthocephalans had ovaries and oval-shaped eggs with an eggshell (77.5 × 17.1 μm), floating within the body cavity of the trunk. In the infected posterior intestine of P. major, the presoma and the anterior part of the metasoma of L. pagrosomi passed through the intestinal wall and infected the intestinal tissue, perforating the loose connective tissue. In the inflammatory connective tissue, collagen and muscle fibers were fragmented and revealed partial necrosis. Lipid drops and eosinophilic granular cells aggregated in the connective tissue of the tissue capsule. In the vicinity of the acanthocephalan, the mucosal epithelia contained hypertrophied nuclei, and the epithelial layer was collapsed. In an extreme case, the mucosal fold was degenerated because of pressure from the acanthocephalan.










Similar content being viewed by others
References
Alava JJ, Aguirre WE (2005) Scanning electron microscopy of Neoechinorhynchus sp. (Acanthocephala: Neoechinorhynchidae), a possible new species of intestinal parasite of the tallfin croaker Micropogonias altipinnis (Günther, 1864). Parasitol Latinoam 60:48–53
Amin OM (1985) Classification. In: Crompton DWT, Kickol BB (eds) Biology of the acanthocephala. Cambridge University Press, Cambridge, pp 1–152
Amin OM, Abdullah SMA, Mhaisen FT (2003) Description of Pomphorhynchus spindletruncatus n. sp. (Acanthocephala: Pomphorhynchidae) from freshwater fishes in northern Iraq, with the erection of a new pomphorhynchid genus, Pyriproboscis n. g., and key to genera of the Pomphorhynchidae and the species of Pomphorhynchus Monticelli, 1905. Syst Parasitol 54:229–235
Bayoumy ME, Abd El-Hady OK, Osman HAM (2006) Site adaptations of Acanthogyrus (Acanthosentis) tilapiae: observations through light and scanning electron microscopy. J Vet Sci 7:339–342
Chaicharn A, Bullock WL (1967) The histopathology of acanthocephalan infections in suckers with observations on the intestinal histology of two species of catostomid fishes. Acta Zool 48:19–42
Dezfuli BS, Lui A, Giari L, Boldrini P, Giovinazzo G (2008) Ultrastructural study on the body surface of the acanthocephalan parasite Dentitruncus truttae in brown trout. Microsc Res Tech 71:230–235
Dural M, Genc E, Yemenicioğlu S, Sangun MK (2010) Accumulation of some heavy metals seasonally in Hysterotylacium aduncum (Nematoda) and its host red sea bream, Pagellus erythrinus (Sparidae), from Gulf of Iskenderun (North-Eastern Mediterranean). Bull Environ Contam Toxicol 84:125–131
Drury RAB, Wallington EA (1980) Carleton's histological technique. Oxford University Press, Oxford, pp 1–520
Edmonds SJ, Smales LR (1992) A new species of Acanthocephala from the greenback flounder, Rhombosolea tapirina Günther, 1982. Trans R Soc S Aust 116:35–38
Eiras JC, Pavanelli GC, Machado MH (1995) Infection of Oxydoras kneri Bleecker, 1862 (Pisces, Doradidae) by the acanthocephalan Paracavisom impudica (Diesing, 1851) Kritcher, 1957. Mem Inst Oswaldo Cruz Rio de Janeiro 90:629–631
George PV, Nadakal AM (1981) Observations on the intestinal pathology of the marine fish Rachycentron canadus infected with the acanthocephalid worm, Serrasentis nadakali. Hydrobiologia 78:59–62
Hatai K, Horita Y, Kubota SS (1987) A histopathological study of longicollosis in cultured red sea bream, Pagrus major. Fish Pathol 22:31–32
Jilek P (1979) Histopathology due to the presence of Gracilisentis gracilisentis in Dorosoma cepedianum. J Fish Biol 14:593–595
McDonough JM, Gleason LM (1981) Histopathology in the rainbow darter, Etheostoma caeruleum, resulting from infections with the acanthocephalans, Pomphorhynchus bulboccoli and Acanthocephalus dirus. J Parasitol 67:403–409
Mehlhorn H (2001) Encyclopedic reference of parasitology: disease, treatment, therapy. Springer, Berlin, pp 10–25
Möller H, Anders K (1986) Disease and parasites of marine fishes. Verlag Möller, Kiel, pp 179–183
Neff BD, Cargnelli LM (2004) Relationships between condition factors, parasite load and paternity in bluegill sunfish, Lepomis macrochirus. Environ Biol Fish 71:297–304
Pichelin S, Smales L, Bray RA (2002) A discussion on the heteracanthocephalidae Petrochenko, 1956 (Acanthocephala: Palaecanthocephala). Syst Parasitol 52:145–152
Roubal FR (1993) Comparative histopathology of Longicollum (Acanthocephala: Pomphorhynchidae) infection in the alimentary tract and spleen of Acanthopagrus australis (Pisces: Sparidae). Int J Parasitol 23:391–397
Ruppert EE, Barnes RD (1994) Invertebrate zoology. Saunders College Publishing, Fort Worth, pp 277–335
Sasal P, Faliex E, De Buron I, Morand S (2001) Sex discriminatory effect of the acanthocephalan Acanthocephaloides propinquus on the gobiid fish Gobius bucchichii. Parasite 8:231–236
Sures B (2003) Accumulation of heavy metals by intestinal helminths in fish: an overview and perspective. Parasitology 126:53–60
Sures B (2004) Fish acanthocephalans of the genus Pomphorhynchus sp. as globally applicable bioindicators for heavy metal pollution in the aquatic environment? Wien Klin Wochenschr 116:19–23
Sures B (2006) How parasitism and pollution affect the physiological homeostasis of aquatic hosts. J Helminthol 80:151–157
Taraschewski H (2008) Acanthocephala. In: Eiras JC, Segner H, Wahli T, Kapoor BG (eds) Fish diseases, vol 2. Science Publishers, Enfield, pp 1025–1062
Wanstall ST, Robotham PW, Thomas JS (1986) Pathological changes induced by Pomphorhynchus laevis Müller (Acanthocephala) in the gut of rainbow trout, Salmo gairdneri Richardson. Z Parasitenkd 72:105–114
Wanstall ST, Thomas JS, Robotham PWJ (1988) The pathology caused by Pomphorhynchus laevis Müller in the alimentary tract of the stone loach, Noemacheilus barbatulus (L.). J Fish Dis 11:511–523
Wayland MT, Gibson DI, Sommerville C (2004) Echinorhynchus salmonis Müller, 1784 (Acanthocephala: Echinorhynchidae) from the Bothnian Bay, Baltic Sea: morphological variability and radial asymmetry of proboscis hooks. Syst Parasitol 58:149–158
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SR., Lee, J.S., Kim, JH. et al. Fine structure of Longicollum pagrosomi (Acanthocephala: Pomphorhynchidae) and intestinal histopathology of the red sea bream, Pagrus major, infected with acanthocephalans. Parasitol Res 109, 175–184 (2011). https://doi.org/10.1007/s00436-010-2241-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2241-z