Abstract
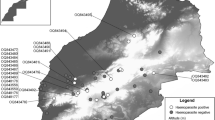
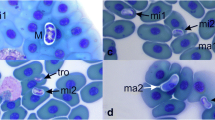
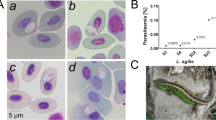
We investigated the occurrence of blood parasites of two lizard species: the common or viviparous lizard (Zootoca vivipara) and the sand lizard (Lacerta agilis) in western Poland. Selected traits of lizard body morphology were studied with respect to the presence and intensity of haematozoan infection in blood samples collected from 218 adult lizards; 88 of the common lizard and 130 of the sand lizard. Haemogregarinid blood parasites were found to be the common parasite of both lizard species in studied locality with prevalence 39.8 (95% CL, 29.5–50.8) for Z. vivipara and 22.3 (95% CL, 15.5–30.4) for L. agilis. Incidence of parasitemia did not differ between sexes and was not correlated with morphological traits or presence of ectoparasites—Ixodes ricinus ticks. However, a significant difference between the two species of lizards was a greater frequency of haemogregarinid parasitism in Z. vivipara.


Similar content being viewed by others
References
Allan BF, Keesing F, Ostfeld RS (2003) Effect of forest fragmentation on lyme disease risk. Conserv Biol 17:267–272
Amo L, Fargallo JA, Martínez-Padilla J, Millán J, López P, Martin J (2005) Prevalence and intensity of blood and intestinal parasites in a field population of a Mediterranean lizard, Lacerta lepida. Parasitol Res 96:413–417
Amo L, Lopez P, Martin J (2004) Prevalence and intensity of haemogregarinid blood parasites in a population of the Iberian rock lizard, Lacerta monticola. Parasitol Res 94:290–293
Antczak M, Hromada M, Grzybek J, Tryjanowski P (2004) Breeding biology of the Great Grey Shrike Lanius excubitor in W Poland. Acta Ornithol 39:9–14
Appleby BM, Anwar MA, Petty SJ (1999) Short-term and long-term effects of food supply on parasite burdens in tawny owls, Strix aluco. Funct Ecol 13:315–321
Arneberg P, Skorping A, Grenfell BT, Read AF (1998) Host densities as determinants of abundance in parasite communities. Proc R Soc Lond B 265:1283–1289
Belliure J, Smith L, Sorci G (2004) Effect of testosterone on T cell mediated immunity in two species of Mediterranean Lacertid lizards. J Exp Zool 301A:411–418
Bennett GF, Cameron M (1974) Seasonal prevalence of avian hematozoa in passeriform birds of Atlantic Canada. Can J Zool 52:1259–1264
Bischoff W (1984) Lacerta agilis Linnaeus, 1758 Zauneidechse. In: Böhme W (ed) Handbuch der Reptilien und Amphibien Europas. Aula Verlag, Weisbaden, pp 23–68
Caudell JN, Whittier J, Conover MR (2002) The effects of haemogregarine-like parasites on brown tree snakes (Boiga irregularis) and slatey-grey snakes (Stegonotus cucullatus) in Queensland, Australia. Int Biodeterior Biodegrad 49:113–119
Corbett KF, Tamarind DL (1979) Conservation of the sand lizard, Lacerta agilis, by habitat management. Brit J Herp 5:799–823
Dowell SF (2001) Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis 7:369–374
Eisen RJ (2000) Variation in life-history traits of Plasmodium mexicanum, a malaria parasite infecting western fence lizards: a longitudinal study. Can J Zool 78:1230–1237
Eisen RJ, Wright NM (2001) Landscape features associated with infection by a malaria parasite (Plasmodium mexicanum) and the importance of multiple scale studies. Parasitology 122:507–513
Ekner A, Majláth I, Majláthová V, Hromada M, Bona M, Antczak M, Bogaczyk M, Tryjanowski P (2008) Densities and morphology of two co-existing lizard species (Lacerta agilis and Zootoca vivipara) in extensively used farmland in Poland. Folia Biol 56:165–171
Fargallo JA, Merino S (2004) Clutch size and haemoparasite species richness in adult and nestling blue tits. Ecoscience 11:168–174
Gustafsson L, Nordling D, Andersson MS, Sheldon BC, Qvarnstrom A (1994) Infectious diseases, reproductive effort and the cost of reproduction in birds. Philos Trans R Soc Lond B 346:323–331
Majláthová V, Majláth I, Hromada M, Tryjanowski P, Bona M, Antczak M, Víchova B, Dzimko S, Mihalca A, Petko B (2008) The role of the sand lizard (Lacerta agilis) in the transmission cycle of Borrelia burgdorferi sensu lato. Int J Med Microbiol 298:161–167
Martin J, Amo L, López P (2008) Parasites and health affect multiple sexual signals in male common wall lizards, Podarcis muralis. Naturwissenschaften 95:293–300
Martin J, Lopez P (1999) An experimental test of the costs of antipredatory refuge use in the wall lizard, Podarcis muralis. Oikos 84:499–505
Minchella DJ (1985) Host life-history variation in response to parasitism. Parasitology 90:205–216
Møller AP (1997) Parasitism and the evolution of host life history. In: Clayton DH, Moore J (eds) Host–parasite evolution: general principles and avian models. Oxford University Press, Oxford, pp 105–127
Nordling D, Andersson M, Zohari S, Gustafsson L (1998) Reproductive effort reduces specific immune response and parasite resistance. Proc R Soc Lond 265:1291–1298
Norris K, Anwar M, Read AF (1994) Reproductive effort influences the prevalence of haematozoan parasites in great tits. J Anim Ecol 63:601–610
Olsen W (1974) Animal parasites. University Park Press, Toronto
Olsson M, Wapstra E, Madsen T, Ujvari B, Rugfelt C (2005) Costly parasite resistance: a genotype-dependent handicap in sand lizards? Biol Lett 1:375–377
Oppliger A, Clobert J (1997) Reduced tail regeneration in the common lizard, Lacerta vivipara, parasited by blood parasites. Funct Ecol 11:652–655
Oppliger A, Clobert J, Lecomte J, Lorenzon P, Boudjemadi K, John-Alder HB (1998) Environmental stress increases the prevalence and intensity of blood parasite infection in the common lizard Lacerta vivipara. Ecol Lett 1:129–138
Price PW (1980) Evolutionary biology of parasites. Princeton University Press, Princeton
Readon JT, Norbury G (2004) Ectoparasite and hemoparasite infections in a diverse temperate lizard assemblage at Macraes Flat, South Island, New Zealand. J Parasitol 90:1274–1278
Reichenow E (1913) Karyolysus lacertae, ein wirtswechselndes Coccidium an der Eidechse Lacerta muralis und der Milbe Liponyssus saurarum. Arb Kaiserlichen Gesundheitsamte 45:317–363
Salkeld DJ, Leonhard S, Girard YA, Hahn N, Mun J, Padgett KA, Lane RS (2008) Identifying the reservoir hosts of the Lyme disease spirochete Borrelia burgdorferi in California: the role of the western gray squirrel (Sciurus griseus). Am J Trop Med Hyg 79:535–540
Salvador A, Veiga JP, Martin J, Lopez P (1997) Testosterone supplementation in subordinate, small male lizards: consequences for aggressiveness, color development, and parasite load. Behav Ecol 8:135–139
Schall JJ (1986) Prevalence and virulence of a haemogregarine parasite of the Aruban whiptail lizard, Cnemidophorus arubensis. J Herpetol 20:318–324
Schall JJ, Marghoob AB (1995) Prevalence of a malarial parasite over time and space: Plasmodium mexicanum in its vertebrate host, the western fence lizard Sceloporus occidentalis. J Anim Ecol 64:177–185
Schall JJ, Pearson AR, Perkins SL (2000) Prevalence of malaria parasites (Plasmodium floridense and Plasmodium azurophilum) infecting a Puerto Rican lizard (Anolis gundlachi): a nine-year study. J Parasitol 86:511–515
Smallridge CJ, Bull CM (2000) Prevalence and intensity of the blood parasite Hemolivia mariae in a field population of the skink Tiquila rugosa. Parasitol Res 86:655–660
Sol D, Jovani R, Torres J (2000) Geographical variation in blood parasites in feral pigeons: the role of vectors. Ecography 23:307–314
Sorci G (1996) Patterns of haemogregarine load, aggregation and prevalence as a function of host age in the lizard Lacerta vivipara. J Parasitol 82:676–678
Telford SR (2008) Hemoparasites of the reptilia. Color atlas and text. CRC Press, Boca Raton
Veiga JP, Salvador A, Merino S, Puerta M (1998) Reproductive effort affects immune response and parasite infection in a lizard: a phenotypic manipulation using testosterone. Oikos 82:313–318
Votýpka J, Simek J, Tryjanowski P (2003) Blood parasites, reproduction and sexual selection in the red-backed shrike (Lanius collurio). Ann Zool Fenn 40:431–439
Acknowledgement
We are very grateful to M. Biskup and M. Bogaczyk for assistance in the field. This work was financially supported by the Scientific Grant Agency of the Ministry of Education of Slovak Republic and the Slovak Academy of Sciences No. 1/0139/08, MH was supported by grant MSM 6007665801, and NN 303 3174 33 by the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Majláthová, V., Majláth, I., Haklová, B. et al. Blood parasites in two co-existing species of lizards (Zootoca vivipara and Lacerta agilis). Parasitol Res 107, 1121–1127 (2010). https://doi.org/10.1007/s00436-010-1981-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1981-0