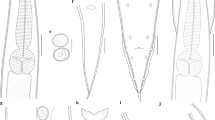
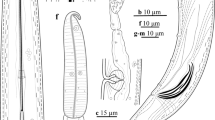
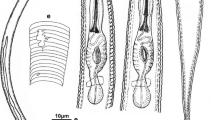
Abstract
In the present study, five species of Contracaecum Railliet & Henry 1912 (Nematoda: Ascaridida), including Contracaecum bancrofti, Contracaecum microcephalum, Contracaecum variegatum, Contracaecum eudyptulae, and Contracaecum ogmorhini, were redescribed using light and scanning electron microscopy. In addition, in order to elucidate their taxonomic status, first and second internal transcribed spacers (ITS-1 and ITS-2, respectively) of nuclear ribosomal DNA of each morphospecies were characterized. Analyses of sequence and morphological data sets suggested that C. bancrofti, infecting the Australian pelican Pelecanus conspicillatus, is a valid species and is distinct from C. micropapillatum reported from pelicans in the northern hemisphere. C. microcephalum from cormorants Phalacrocorax melanoleucos and C. variegatum from the darter Anhinga melanogaster and from P. conspicillatus as well as C. eudyptulae from the little penguin Eudyptula minor were also considered as distinct species, which can be differentiated from one another morphologically based on the lengths of spicules and genetically based on the sequences of ITS-1 and ITS-2. Comparison of sequence data of ITS-1 and ITS-2 for the members of C. ogmorhini sensu lato from pinnipeds with those of previous studies suggested that only ITS-2 can be used for differentiation between C. ogmorhini sensu stricto and Contracaecum margolisi, occurring in the southern and northern hemispheres, respectively. Analyses of the ITS-1 and ITS-2 sequence data of Contracaecum spp. in the present study supported the distinction among species of Contracaecum based on morphological data and were useful in confirming the taxonomic status of individual species in Australia.













Similar content being viewed by others
References
Anderson RC (2000) Nematode parasites of vertebrates, their development and transmission, 2nd edn. CAB International, Wallingford, p 650
Baylis HA (1920) On the classification of the Ascaridae. I. The systematic value of certain characters of the alimentary canal. Parasitology 12:253–264
Campana-Rouget Y, Paulian P (1960) Mise en synonymie de deux espèces de Contracaecum, parasites de mammifères marins. Ann Parasitol Hum Comp 35:191–192
Chilton NB, Gasser RB, Beveridge I (1995) Differences in a ribosomal DNA sequence of morphologically indistinguishable species within the Hypodontus macropi complex (Nematoda: Strongyloidea). Int J Parasitol 25:647–651
Cram EB (1927) Bird parasites of the nematode suborders Strongylata, Ascaridata, and Spirurata. Smithsonian Institution. United States National Museum. Bulletin no. 10:465 pp
Dailey MD (1975) The distribution and intraspecific variation of helminth parasites in pinnipeds. Rapports et Proces-Verbaux des Reunions, Conseil International pour l'Exploration de la Mer [Proceeding of the Symposium on the Biology of the Seal, 14–17 Aug. 1972, Guelph, Canada.] 169:338–352
Fagerholm HP (1991) Systematic implications of male caudal morphology in ascaridoid nematode parasites. Syst Parasitol 19:215–228
Fagerholm HP, Gibson DI (1987) A redescription of the pinniped parasite Contracaecum ogmorhini (Nematoda, Ascaridoidea), with an assessment of its antiboreal circumpolar distribution. Zool Scr 16:19–24
Fagerholm HP, Overstreet RM, Humphrey-Smith I (1996) Contracaecum magnipapillatum (Nematoda, Ascaridoidea): resurrection and pathogenic effect of a common parasite from the proventriculus of Anous minutus from the Great Barrier Reef, with a note on C. variegatum. Helminthologia 33:195–207
Gibson DI (1983) The systematics of ascaridoid nematodes-a current assessment. In: Stone AR, Platt HM, Khalil LF (eds) Concepts in nematode systematics. Systematics Association Special Volume No. 22. Academic, London, pp 321–338
Hartwich G (1957) Zur systematik der Nematoden-superfamilie Ascaridoidea. Zoologische Jahrebucher 85:211–252
Hartwich G (1964) Revision der vogelparasitischen nematoden mitteleuropas, II. Di Gattung Contracaecum Railliet & Henry, 1912. Mitteilungen aus dei Zoologisches Museum Berlin 40:15–53
Hartwich G (1974) Keys to genera of the Ascaridoidea. In: Anderson RC, Chabaud AG, Willmott S (eds) CIH keys to the nematode parasites of vertebrates. Commonwealth Agricultural Bureaux No. 2, pp 1–15
Hugot JP, Morand S, Vassart M (1991) Morphological study of Contracaecum magnicollare (Nematoda: Anisakidae) from Anous minutus (Aves, Laridae). Syst Parasitol 20:229–236
Johnston TH (1937) Entozoa from Australian hair seal. Proceeding of the Linnean Society of New South Wales 62:9–16
Johnston TH (1938) Parasitic Nematoda. Scientific Report of Australian Antarct Expedition, Ser. C, 10:1–31
Johnston TH, Mawson PM (1941a) Additional nematodes from Australian birds. Trans Roy Soc S Aust 65:254–262
Johnston TH, Mawson PM (1941b) Ascaroid nematodes from Australian birds. Trans Roy Soc S Aust 65:110–115
Johnston TH, Mawson PM (1941c) Nematodes from Australian marine mammals. Rec South Aust Mus 6:429–434
Johnston TH, Mawson PM (1941d) Some nematode parasites from Australian birds. Proceeding of the Linnean Society of New South Wales 64:250–256
Johnston TH, Mawson PM (1941e) Some nematodes from Australian birds of prey. Trans Roy Soc S Aust 65:30–35
Johnston TH, Mawson PM (1941f) Some parasitic nematodes in the collection of the Australian museum. Rec Aust Mus 21:9–16
Johnston TH, Mawson PM (1942a) Nematodes from Australian albatrosses and petrels. Trans Roy Soc S Aust 66:66–70
Johnston TH, Mawson PM (1942b) Remarks on some parasitic nematodes. Rec South Aust Mus 7:183–186
Johnston TH, Mawson PM (1942c) Some new and known Australian parasitic nematodes. Proceeding of the Linnean Society of New South Wales 97:90–94
Johnston TH, Mawson PM (1945) Parasitic nematodes. British, Australian and New Zealand Antarctic Research Expedition. Reports, Series B 5:73–160
Johnston TH, Mawson PM (1947) Some avian and fish nematodes, chiefly from Tailem Bend, South Australia. Rec South Aust Mus 8:547–553
Johnston TH, Mawson PM (1949) Some nematodes from Australian hosts, together with a note on Rhabditis allgeni. Trans Roy Soc S Aust 73:63–71
Johnston TH, Mawson PM (1952) Some nematodes from Australian birds and mammals. Trans Roy Soc S Aust 75:30–37
Johnston TH, Mawson PM (1953) Parasitic nematodes and trematodes from Campbell and Auckland Islands (Cape Expedition). Rec Dom Mus 2:63–71
Lent H, Freitas JFTD (1948) Uma colecao de Nematodeos, parasitos de vertebrados, do Musea de Historia Natural de Montevideo. Mem Inst Oswaldo Cruz 46:1–71
Linstow O (1907) Nematodes of Scottish National Antarctic Expedition. Proc Roy Soc Edinb 26:464–472
Mattiucci S, Cianchi R, Nascetti G, Paggi L, Sardella N, Timmi J, Webb SC, Bastida R, Rodriquez D, Bullini L (2003) Genetic evidence for two sibling species within Contracaecum ogmorhini Johnston & Mawson, 1941 (Nematoda: Anisakidae) from otariid seals of boreal and austral regions. Syst Parasitol 54:13–23
Mattiucci S, Paoletti M, Webb SC, Sardella N, Timi JT, Berland B, Nascetti G (2008) Genetic relationships among species of Contracaecum Railliet & Henry, 1912 and Phocascaris Host, 1932 (Nematoda: Anisakidae) from pinnipeds inferred from mitochondrial cox2 sequences, and congruence with allozyme data. Parasite 15:408–419
Mawson P (1953) Parasitic Nematoda collected by the Australian National Antarctic Research Expedition: Heard Island and Macquarie Island, 1948–1951. Parasitology 43:291–297
Mawson P (1969) Some nematodes from Australian gulls and terns. J Fish Res Board Can 26:1103–1111
Mawson PM, Angel M, Edmonds SJ (1986) A checklist of helminths from Australian birds. Rec South Aust Mus 19:219–325
Mozgovoy AA (1953) Ascaridata of animals and man and the diseases caused by them. In: Fundamentals of nematodology, vol 2. Translated from Russian by the Israel Program for Scientific Translation, 1968, pp 8–42
Nadler SA, D'Amelio S, Dailey M, Paggi L, Siu S, Sakanari JA (2005) Molecular phylogenetics and diagnosis of Anisakis, Pseudoterranova and Contracaecum from northern Pacific marine mammals. J Parasitol 91:1413–1429
Nagasawa K, Barus V, Tenora F, Oka N (1999) Contracaecum variegatum (Nematoda: Anisakidae) from the Pacific diver (Gavia pacifica) in Japan. Biogeography 1:107–110
Nascetti G, Cianchi R, Mattiucci S, D'Amelio S, Orecchia P, Paggi L, Brattey J, Berland B, Smith JW, Bullini L (1993) Three sibling species within Contracaecum osculatum (Nematoda, Ascaridida, Ascaridoidea) from the atlantic arctic-boreal region: reproductive isolation and host preferences. Int J Parasitol 23:105–120
Norman R (2005) Parasitic diseases of the little penguin, Eudyptula minor (Forster, 1781), with emphasis on nematodes of the genus Contracaecum Railliet & Henry, 1912 (Anisakidae). PhD Thesis. Faculty of Veterinary Science, The University of Melbourne. 693 pp
Orecchia P, Mattiucci S, D'Amelio S, Paggi L, Plotz J, Cianchi R, Nascetti G, Arduino P, Bullini L (1994) Two new members in the Contracaecum osculatum complex (Nematoda: Ascaridoidea) from the Antarctic. Int J Parasitol 24:367–377
Rudolphi CA (1809) Entozoorum sive vermium intestinalium. Historia Naturalis. Vol. 2. Amsteodami. 457 pp
Shamsi S, Gasser RB, Beveridge I (2008) Contracaecum pyripapillatum n. sp. (Nematoda: Anisakidae) and a description of C. multipapillatum (von Drasche, 1882) from the Australian pelican, Pelecanus conspicillatus. Parasitol Res 103:1031–1039
Sprent JFA (1983) Observations on the systematics of ascaridoid nematodes. In: Stone AR, Platt HM, Khalil LF (eds) Concepts in nematode systematics. Systematic Association Special Volume No. 22. Academic, London, pp 303–319
Thompson JD, Gibson TJ, Plewniac F, Jeanmougin F, Higgins DG (1997) The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Yamaguti S (1935) Studies on the helminth fauna of Japan. Part 12. Avian nematodes, I. Jpn J Zool 6:403–431
Yamaguti S (1961) Systema helminthum, vol. 3. The nematodes of vertebrates. Part II. Interscience, New York, p 1575
Zhu X, Gasser RB, Podolska M, Chilton NB (1998) Characterisation of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. Int J Parasitol 28:1911–1921
Zhu X, Gasser RB, Jacobs DE, Hung GC, Chilton NB (2000) Relationships among some ascaridoid nematodes based on ribosomal DNA sequence data. Parasitol Res 86:738–744
Zhu XQ, Gasser RB, Chilton NB, Jacobs DE (2001) Molecular approaches for studying ascaridoid nematodes with zoonotic potential, with an emphasis on Toxocara species. J Helminthol 75:101–108
Acknowledgments
The authors thank Professors Lia Paggi and Hans-Peter Fagerholm for discussions. Shokoofeh Shamsi was a grateful recipient of scholarships from the University of Melbourne.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nucleotide sequence data reported in this paper are available in the GenBank database under the accession numbers EU839566–EU839573, FM177523–FM177578, and FM177880–FM177888.
Rights and permissions
About this article
Cite this article
Shamsi, S., Norman, R., Gasser, R. et al. Redescription and genetic characterization of selected Contracaecum spp. (Nematoda: Anisakidae) from various hosts in Australia. Parasitol Res 104, 1507–1525 (2009). https://doi.org/10.1007/s00436-009-1357-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1357-5