Abstract
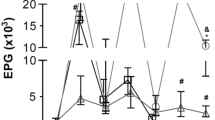
The Sarcocystidae comprise a diverse, monophyletic apicomplexan parasite family, most of whose members form intracellular cysts in their intermediate hosts. The extent of pathology associated with such cyst formation can range widely. We currently lack experimental animal models for many of these infections. Here we explored dexamethasone treatment as a means to render outbred mice susceptible to Besnoitia darlingi infection and demonstrated that this approach allows viable parasites to be subsequently isolated from these mice and maintained in tissue culture. Besnoitia bradyzoites recovered from crushed cysts derived from naturally infected opossums (Didelphis virginiana) replicated and reproduced the development of besnoitiosis in mice treated with dexamethasone (0.5 mg/ml drinking water) daily for 12 days post infection (DPI). Isolates recovered from the peritoneal exudates of these mice were viable and were maintained in long-term tissue cultures. In contrast, control mice given saline without dexamethasone and challenged with similar bradyzoites remained clinically normal for up to 70 DPI. An additional group of mice challenged with the same inoculum of bradyzoites and given dexamethasone at the same concentration and treated with sulfadiazine (1 mg/ml drinking water) daily for 12 DPI also remained normal for up to 70 DPI. Severe disease developed more rapidly in dexamethasone-treated mice inoculated with culture-derived B. darlingi tachyzoites than in those inoculated with cyst-derived bradyzoites. B. darlingi tachyzoite-infected, untreated control mice developed signs of illness at 18 DPI. In contrast, mice treated with sulfadiazine showed no clinical signs up to 50 DPI. Although dexamethasone treatment was required to establish B. darlingi infection in outbred mice inoculated with opossum-derived B. darlingi bradyzoites, no such treatment was required for mice inoculated with culture-derived B. darlingi tachyzoites. Finally, sulfadiazine was highly effective in protecting mice from infection with the tachyzoite stage of B. darlingi.



Similar content being viewed by others
References
Boy MG, Galligan DT, Divers TJ (1990) Protozoal encephalomyelitis in horses: 82 cases (1972–1986). J Am Vet Med Assoc 196:632–634
Cox WI, Holbrook NJ, Grasso RJ, Specter S, Friedman H (1982) Suppression of the natural killer cell activity of murine spleen cell cultures by dexamethasone (41489). Proc Soc Exp Biol Med 171:146–150
Cutler TJ, MacKay RJ, Ginn PE, Gillis K, Tanhauser SM, LeRay V, Dame JB, Greiner EC (2001) Immunoconversion against Sarcocystis neurona in normal and dexamethasone-treated horses challenged with S. neurona sporocysts. Vet Parasitol 95:197–210
Darius AK, Mehlhorn H, Heydorn AO (2004) Effects of toltrazuril and ponazuril on Hammondia heydorni (syn. Neospora caninum) infections in mice. Parasitol Res 92:520–522
Dubey JP, Davis GW, Koestner A, Kiryu K (1974) Equine encephalomyelitis due to a protozoan parasite resembling Toxoplasma gondii. J Am Vet Med Assoc 165:249–255
Dubey JP, Lindsay DS, Rosenthal BM, Sreekumar C, Hill DE, Shen SK, Kwok OCH, Rickard LG, Black SS, Rashmir-Raven A (2002) Establishment of Besnoitia darlingi from opossums (Didelphis virginiana) in experimental intermediate and definitive hosts, propagation in cell culture, and description of ultrastructural and genetic characteristics. Int J Parasitol 32:1053–1064
Elsheikha HM, Mansfield LS (2004) Determination of the activity of sulfadiazine against Besnoitia darlingi tachyzoites in cultured cells. Parasitol Res 93:423–426
Elsheikha HM, Mansfield LS, Fitzgerald SD, Saeed MA (2003) Prevalence and tissue distribution of Besnoitia darlingi cysts in the Virginia opossum (Didelphis virginiana) in Michigan. Vet Parasitol 115:321–327
Elsheikha HM, Fitzgerald SD, Rosenthal BM, Mansfield LS (2004) Concurrent presence of Sarcocystis neurona sporocysts, Besnoitia darlingi tissue cysts, and Sarcocystis inghami sarcocysts in naturally infected opossums (Didelphis virginiana). J Vet Diagn Invest 16:352–356
Fenger CK, Granstrom DE, Gajadhar AA, Williams NM, McCrillis SA, Stamper S, Langemeier JL, Dubey JP (1997) Experimental induction of equine protozoal myeloencephalitis in horses using Sarcocystis sp. sporocysts from the opossum (Didelphis virginiana). Vet Parasitol 68:199–213
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: Molecular Evolutionary Genetics Analysis software. Arizona State University, Tempe
Pan J, Ju D, Wang Q, Zhang M, Xia D, Zhang L, Yu H, Cao X (2001) Dexamethasone inhibits the antigen presentation of dendritic cells in MHC class II pathway. Immunol Lett 76:153–161
Paperna I, Lainson R (2001) Light microscopical structure and ultrastructure of a Besnoitia sp. in the naturally infected lizard Ameiva ameiva (Teiidae) from north Brazil, and in experimentally infected mice. Parasitology 123: 247–255
Schneider CR (1976) The distribution of lizard besnoitiosis in Panama, and its transfer to mice. J Protozool 14:674–678
Senaud J, Mehlhorn H, Scholtyseck E (1974) Besnoitia jellisoni in macrophages and cysts from experimentally infected laboratory mice. J Protozool 21:715–720
Shkap V, Pipano E, Greenblatt C (1987) Cultivation of Besnoitia besnoiti and evaluation of susceptibility of laboratory animals to cultured parasites. Vet Parasitol 23:169–178
Smith DD, Frenkel JK (1977) Besnoitia darlingi (Protozoa: Toxoplasmatinae): cyclic transmission by cats. J Parasitol 63:1066–1071
Tanhauser SM, Yowell CA, Cutler TJ, Greiner EC, MacKay RJ, Dame JB (1999) Multiple DNA markers differentiate Sarcocystis neurona and Sarcocystis falcatula. J Parasitol 85:221–228
Acknowledgements
We thank Dr. Frances Kennedy from the Diagnostic Center for Population and Animal Health, Michigan State University (MSU) for her kind assistance with pathology and Dr. Shirly Owens from the Center for Advanced Microscopy, MSU for her kind help with illustrations. We also thank Abimbola Odukoyu and Tran Ta for technical help with the PCR and sequencing experiments. This study was performed in accordance to the law and regulations of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elsheikha, H.M., Rosenthal, B.M. & Mansfield, L.S. Dexamethasone treatment induces susceptibility of outbred Webster mice to experimental infection with Besnoitia darlingi isolated from opossums (Didelphis virginiana). Parasitol Res 95, 413–419 (2005). https://doi.org/10.1007/s00436-004-1286-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-004-1286-2