Abstract
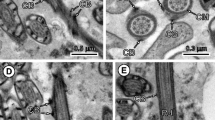
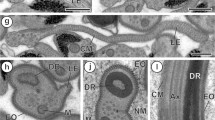
Strobilae from Taenia crassiceps (WFU strain) were obtained from outbred hamsters (Mesocricetus auratus) by feeding them viable metacestodes maintained by intraperitoneal passage in female Balb/c mice. Mature and gravid proglottids from strobilae were recovered from hamster intestines and fixed for light and electron microscopy. By light microscopy, the expected structure of taeniid proglottids was observed. Ultrastructural analysis of ten proglottids showed that testicular follicles and vas deferens contained filiform spermatids, with a single axoneme, and an elongated helicoidal nucleus inserted between the axoneme and the spiraled cortical microtubules. At the apical cone, a single crest-like body was found and mature spermatids also exhibited transverse intracytoplasmic walls. The morphology and characters of the spermatids in T. crassiceps conform to type III spermiogenesis, which has been described in other taeniids.




Similar content being viewed by others
References
Ba CT, Marchand B (1994a) Ultrastructure of spermiogenesis and the spermatozoon of Raillietina (Raillietina) tunetensis (Cyclophyllidea, Davaineidae), intestinal parasite of turtle doves in Senegal. Int J Parasitol 24:237–248
Ba CT, Marchand B (1994b) Ultrastructure of spermiogenesis and the spermatozoon Mathevotaenia herpestis (Cestoda), intestinal parasite of Atelerix albiventrix in Senegal. Acta Zool (Stockh) 75:167–175
Euzet L, Swiderski Z, Mokhtar-Maamouri F (1981) Ultrastructure comparée du spermatozoïde des Cestodes. Relations avec la phylogénèse. Ann Parasitol Hum Comp 56:247–259
Everhart ME, Kuhn RE, Zelmer DA (2004) Intrapopulation dynamics of a wild strain of Taenia crassiceps (WFU) (Cestoda: Taeniidae) in Balb/cJ mice. J Parasitol (in press)
Featherstone DW (1971) Taenia hydatigena. III. Light and electron microscope study of spermatogenesis. Z Parasitenkd 37:148–168
Freeman RS (1962) Studies on the biology of Taenia crassiceps (Zeder, 1800) Rudolphi,1810 (Cestoda). Can J Zool 40:989–990
Hidalgo C, Miquel J, Torres J, Marchand B (2000) Ultrastructural study of spermiogenesis and the spermatozoon in Catenotaenia pusilla, an intestinal parasite of Mus musculus. J Helminthol 74:73–81
Justine JL (1998) Spermatozoa as phylogenetic characters for the Eucestoda. J Parasitol 84:385–408
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137A
Kitaoka M, Oku Y, Okamoto M, Kamiya M (1990) Development and sexual maturation of Taenia crassiceps (Cestoda) in golden hamster. J Parasitol 76:399–402
Lillie RD (1954) Histopathologic technic and practical histochemistry. McGraw-Hill, New York
Miquel J, Ba CT, Marchand B (1998) Ultrastructure of spermiogenesis of Dipylidium caninum (Cestoda, Cyclophyllidea, Dipylidiidae), an intestinal parasite of Canis familiaris. Int J Parasitol 28:1453–1458
Miquel J, Hidalgo C, Feliu C, Marchand B (2000) Spermatozoon ultrastructure of Taenia mustelae (Cestoda, Taeniidae), an intestinal parasite of the weasel, Mustela nivalis (Carnivora). Invert Reprod Dev 38:43–51
Miyaji S, Oku Y, Kamiya M, Okamoto M, Ohbayashi M, Uchida A, Rausch RL (1990) Growth of a Japanese isolate of Taenia crassiceps in intermediate and definitive hosts. Parasitol Res 76:351–354
Ndiaye PI, Miquel J, Marchand B (2002) Ultrastructure of spermiogenesis and spermatozoa of Taenia parva Baer, 1926 (Cestoda, Cyclophyllidea, Taeniidae), a parasite of the common genet. Parasitol Res 89:34–43
Ndiaye PI, Agostini S, Miquel J, Marchand B (2003) Ultrastructure of spermiogenesis and spermatozoon in the genus Joyeuxiella Fuhrmann, 1935 (Cestoda, Cyclophyllidea, Dipylidiidae): comparative analysis of J. echinorhynchoides (Sonsino, 1889) and J. pasqualei (Diamare, 1893). Parasitol Res 91:175–186
Sato H, Kamiya M (1989) Viable egg production of Taenia crassiceps developed in the intestine of prednisolone-treated golden hamsters. Jpn J Parasitol 38:46–53
Sato H, Kamiya M (1990) Establishment, development and fecundity of Taenia crassiceps in the intestine of prednisolone-treated Mongolian gerbils and inbred mice. J Helminthol 64:217–222
Sato H, Kamiya H, Oku Y, Kamiya M (1994). Infection course of the strobilar stage of Taenia crassiceps in golden hamster, with reference to host response. Parasitol Res. 80:99–103
Smith JK, Esch GW, Kuhn RE (1972) Growth and development of larval Taenia crassiceps (Cestoda). I. Aneuploidy in the anomalous ORF strain. Int J Parasitol 2:262–263
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31
Terrazas LI, Bojalil R, Govezensky T, Larralde C (1998) Shift from an early protective Th1-type immune response to a late permissive Th2-type response in murine cysticercosis (Taenia crassiceps). Parasitology 84:74–81
Toenjes SA, Kuhn RE (2003) The initial immune response during experimental cysticercosis is of the mixed Th1/Th2 type. Parasitol Res 89:407–412
Willms K, Caro JA, Robert L (2003) Ultrastructure of spermatogonia and spermatocyte lobules in Taenia solium strobilae (Cestoda, Cyclophyllidea, Taeniidae) from golden hamsters. Parasitol Res 90:479–488
Acknowledgements
The authors thank Laura Aguilar (M.Sc.) for her help in the preparation of whole mount specimens. This work was supported in part by funds from the Consejo Nacional de Ciencia y Tecnología, Mexico (no. L0026). The experiments described here comply with the current laws of Science and Technology in Mexico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willms, K., Robert, L., Jiménez, J.A. et al. Ultrastructure of spermiogenesis and the spermatozoon in Taenia crassiceps strobilae WFU strain (Cestoda, Cyclophyllidea, Taeniidae) from golden hamsters. Parasitol Res 93, 262–267 (2004). https://doi.org/10.1007/s00436-004-1125-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-004-1125-5