Abstract
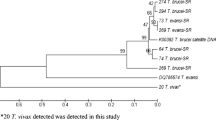
A total of 20 random primers (10-mers) were used to amplify RAPD markers from the genomic DNA of four Trypanosoma brucei stocks from East and West Africa, four T. evansi stocks from Africa, Asia and South America and one T. equiperdum stock from Asia. Between 65 and 88 reproducible fragments ranging from 0.25 to 2.15 kb were generated from these stocks depending on the stock/primer combination. The similarity coefficient (SC) among the stocks of T. brucei from Kenya, Nigeria, Tanzania and Zambia ranged from 62.9% to 74.0% (average: 67.6%). The SC among the stocks of T. evansi from Kenya, China and Brazil was 76.4%–95.5% (average: 86.4%), while the SC between T. evansi stock from China and Brazil was 95.5%. For T. evansi and T. equiperdum, the SC among the stocks ranged from 81.2% to 94.4% (average: 87.6%). As for the SC among the stocks of T. brucei and T. evansi, it was found to be from 54.7% to 80.3% (average: 68.0%) and the SC among stocks of T. brucei and T. equiperdum was from 59.4% to 76.9% (average: 68.1%). Our results indicate that the stocks of T. evansi from China and from Brazil are more closely related to the stock of T. equiperdum from China than to the stocks of T. evansi isolated from Kenya and to the stocks of T. brucei. In addition, our results further support the hypothesis that T. evansi stocks from China and Brazil could have arisen from a single lineage. The possible evolution of T. evansi and T. equiperdum is also discussed.

Similar content being viewed by others
References
Artama WT, Agey MW, Donelson JE (1992) DNA comparison of Trypanosoma evansi from Indonesia and Trypanosoma brucei spp. Parasitology104:64–74
Barral V, This P, Imbert-Establet D, Combes C, Delseny M (1993) Genetic variability and evolution of Schistosoma genome analyzed by using random amplified polymorphic DNA markers. Mol Biochem Parasitol 59:211–222
Basagoudanavar SH, Rao JR, Singh RK, Butchaiah G (1999) Random amplification of polymorphic DNA fingerprinting of Trypanosoma evansi. Vet Res Commun 23:249–255
Biteau N, Bringaud F, Gibson W, Truc P, Baltz T (2000) Characterization of Trypanozoon isolates using a repeated coding sequence and microsatellite markers. Mol Biochem Parasitol 105:185–201
Boid R (1988) Isoenzyme characterisation of 15 stocks Trypanosoma evansi isolated from camels in the Sudan. Trop Med Parasitol 39:45–50
Borst P, Fase-Fowler F, Gibson WC (1987) Kinetoplast DNA of Trypanosoma evansi. Mol Biochem Parasitol 23:31–38
Brisse S, Barnabé C, Tibayrenc M (2000) Identification of six Trypanosoma cruzi phylogenetic lineages by random amplified polymorphic DNA and multilocus enzyme electrophoresis. Int J Parasitol 30:35–44
Brun R, Hecker H, Lun ZR (1998) Trypanosoma evansi and T. equiperdum: distribution, biology, treatment and phylogenetic relationship. Vet Parasitol 79:95–107
Clare F (2003) Characterization of pathogenic trypanosomes. How does Tryapnosoma equiperdum fit into the Trypanozoon group? PhD thesis, Katholieke Universiteit, Leuven
Clare F, Agboo EEC, Radwanska M, Baltz T, De Waal DT, Goddeeris BM, Buscher P (2003) How does Trypanosoma equiperdum fit into the Trypanozoon group? A cluster analysis by random amplified polymorphic DNA (RAPD) and the multiplex-endonuclease genotyping approach. Parasitology 126:425–431
Frasch ACC, Hajduk SL, Hoeijmakers JHJ, Borst P, Brunel F, Davison J (1980) The kinetoplast DNA of Trypanosoma equiperdum. Biochem Biophys Acta 607:397–410
Gibson WC, Marshall TF de C, Godfrey DG (1980) Numerical analysis of enzyme polymorphism: a new approach to the epidemiology and taxonomy of trypanosomes of the subgenus Trypanozoon. Adv Parasitol 18:175–246
Gibson WC, Wilson AJ, Moloo SK (1983) Characterization of Trypanosoma (Trypanozoon) evansi from camels in Kenya using isoenzyme electrophoresis. Res Vet Sci 34:114–118
Hedrick P (1992) Shooting the RAPDs. Nature 355:679–680
Hide G (1997) The molecular epidemiology of Trypanosomatids. In: Hide G, Mottram JC, Coombs GH, Holmes PH (eds) Trypanosomiasis and leishmaniasis. CAB International, Wallingford, pp 289–303
Hoare CA (1972) The trypanosomes of mammals. Blackwell, Oxford
Kaminsky R, Schmid C, Lun ZR (1997) Susceptibility of dyskinetoplastic Trypanosoma evansi and T. equiperdum to isometamidium chloride. Parasitol Res 83:816–818
Kanmogne GD, Stevens, JR, Asonganyi T, Gibson WC (1996) Genetic heterogeneity in the Trypanosoma brucei gambiense genome analysed by random amplification of polymorphic DNA. Parasitol Res 82:535–541
Lanham SM, Godfrey DG (1970) Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol 28:521–534
Luckins AG (1988) Trypanosoma evansi in Asia. Parasitol Today 4:137–142
Lun ZR, Lu LX (1994) Detection of kinetoplast DNA from Trypanosoma evansi by PCR. Chin Sci Bull 39:1310–1314
Lun ZR, Desser SS (1995) Is the broad range of host geographical distribution of Trypanosoma evansi attributable to the loss of maxicircle kinetoplast DNA? Parasitol Today 11:131–133
Lun ZR, Desser SS (1996) Analysis of isolates within species of anuran trypanosomes using random amplified polymorphic DNA. Parasitol Res 82:22–27
Lun ZR, Allinghan R, Brun R, Lanham SM (1992a) The isoenzyme characteristics of Trypanosoma evansi and Trypanosoma equiperdum isolated from domestic stocks in China. Ann Trop Med Parasitol 86:333–340
Lun ZR, Brun R, Gibson W (1992b) Kinetoplast DNA and molecular karyotype of Trypanosoma evansi and Trypanosoma equiperdum from China. Mol Biochem Parasitol 50:189–196
Lun ZR, Fang Y, Wang CJ, Brun R (1993) Trypanosomiasis of domestic animals in China. Parasitol Today 9:41–45
Mathieu-Daude F, Stevens JR, Welsh J, Tibayrenc M, McClelland M (1995) Genetic diversity and population structure of Trypanosoma brucei: clonality versus sexuality. Mol Biochem Parasitol 72:89–101
Nei M, Li WH (1979) Mathematical model for studying genetic variant in term of restriction endonuclease. Proc Nat Acad Sci U S A 76:5269–5273
Njagu Z, Mihok S, Kokwaro E, Verloo D (1999) Isolation of Trypanosoma brucei from the monitor lizard (Varanus niloticus) in an endemic focus of Rhodesian sleeping sickness in Kenya. Acta Trop 72:137–148
Ou YC, Baltz T (1991) Kinetoplast DNA analysis of four Trypanosoma evansi strains. Mol Biochem Parasitol 46:97–102
Reid SA (2002) Trypanosoma evansi control and containment in Australasia. Trends Parasitol 18:219–224
Shu HH, Stuart K (1994) Mitochondrial transcripts are processed but are not edited normally in Trypanosoma equiperdum (ATCC 30019) which has kDNA sequence deletion and duplication. Nucleic Acids Res 22:1696–1670
Songa EB, Paindavoin P, Wittouck E, Viseshakul N, Muldermans S, Steinert M, Hamers R (1990) Evidence for kinetoplast and nuclear DNA homogeneity in Trypanosoma evansi isolates. Mol Biochem Parasitol 43:167–180
Steindel M, Neto ED, Pinto CJC, Grisard EC, Menezes CLP, Murta SMF, Simpson AJG, Romanha AJ (1994) Randomly amplified polymorphic DNA (RAPD) and isoenzyme analysis of Trypanosoma rangeli strains. J Eukaryot Microbiol 41:261–267
Stevens JR, Numes VLB, Lanham SM, Oshiro ET (1989) Isoenzyme characterization of Trypanosoma evansi isolated from capybaras and dogs in Brazil. Acta Trop 46:213–222
Stevens JR, Noyes HA, Schofield CJ, Gibson W (2001) The molecular evolution of trypanosomatidae. Adv Parasitol 48:1–56
Truc P, Tibayrenc M (1993) Population genetics of Trypanosoma brucei in central Africa: taxonomic and epidemiological significance. Parasitology 106:137–149.
Vanácová S, Tachezy J, Kulda J, Flegr J (1997) Characterization of trichomonad species and strains by PCR fingerprinting. J Eukaryot Microbiol 44:545–552
Van Belkun A, Homan W, Limper L, Quint WGV (1993) Genotyping isolates and clones of Giardia duodenalis by polymerase chain reaction: implication for the detection genetic variation among protozoa parasite species. Mol Biochem Parasitol 61:69–78
Van der Ploeg LHT, Bernards A, Rijsewijk F, Borst P (1982) Characterisation of the DNA duplication-transposition that control the expression of two genes for variant surface glycoproteins in Trypanosoma brucei. Nucleic Acids Res 10:593–609
Ventura RM, Takata CSA, Silva RAMS, Nues VL, Takeda, GF, Teixeira MMG (2000) Molecular and morphological studies of Brazilian Trypanosoma evansi stocks: the total absence of kDNA in trypanosomes from both laboratory stocks and naturally infected domestic and wild mammals. J Parasitol 86:1289–1298
Ventura RM, Takeda GF, Silva RAMS, Nunes VLB, Buck GA, Teixeira MMG (2002) Genetic relatedness among Trypanosoma evansi stocks by random amplification of polymorphic DNA and evaluation of a synapomorphic DNA fragment for species-specific diagnosis. Int J Parasitol 32:53–63
Waitumbi JN, Murphy NB (1993) Inter- and intraspecies differentiation of trypanosomes by genomic fingerprinting with arbitrary primers. Mol Biochem Parasitol 58:181–186
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers useful as genetic markers. Nucleic Acid Res 18:6531–6535
Zhang ZQ, Baltz T (1994) Identification of Trypanosoma evansi, Trypanosoma equiperdum and Trypanosoma brucei using repetitive DNA probes. Vet Parasitol 53:197–208
Acknowledgements
The authors thank Dr. A. Luckins for a critical review of the manuscript and Dr. W. Gibson for providing the DNA of T. evansi from Brazil and Africa. This work was supported by grants from the Zhongshan University( no. 3253280) and the National Natural Science Foundation of China (no. 30170124).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lun, ZR., Li, AX., Chen, XG. et al. Molecular profiles of Trypanosoma brucei, T. evansi and T. equiperdum stocks revealed by the random amplified polymorphic DNA method. Parasitol Res 92, 335–340 (2004). https://doi.org/10.1007/s00436-003-1054-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-003-1054-8