Abstract
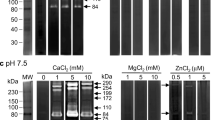
Inhibitor sensitivity assays using azocaesin and FTC-caesin as substrates showed that the excretory/secretory (E/S) products of the infective-stage larvae of Trichinella spiralis contained serine, metallo-, cysteine and aspartic proteinases. The activity of the metallo-proteinase was zinc ion dependent (within a range of ZnSO4 concentrations). Gelatin-substrate gel electrophoresis revealed two bands of molecular mass 48 and 58 kDa which were sensitive to the metallo-proteinase inhibitor EDTA. The former peptide was probably a cleavage product of the latter. The authenticity of the 58 kDa metallo-proteinase as an E/S product was confirmed by immunoprecipitation. Using PCR and RACE reactions, a complete nucleotide sequence of the metallo-proteinase gene was obtained. It comprised 2,223 bp with an open reading frame encoding 604 amino acid residues. The 3′ untranslated region consisted of 352 bp, including a polyadenylation signal AATAA. A consensus catalytic zinc-binding motif was present. The conserved domains suggest that the cloned metallo-proteinase belongs to the astacin family and occurs as a single copy gene with 11 introns and 10 exons. Cluster analysis showed that the sequence of the metallo-proteinase gene of T. spiralis resembles those of Caenorhabdites elegans and Strongyloides stercoralis.










Similar content being viewed by others
References
Baramova E, Foidart JM (1995) Matrix metalloproteinase family. Cell Biol Int 19:239–242
Breathnach R, Chambon P (1981) Organization and expression of eukaryotic split genes coding for proteins. Annu Rev Biochem 50:349–383
Campbell WC (1983) Historical introduction. In: Campbell WC (ed) Trichinella and trichinellosis. Plenum Press, New York, pp 1–30
Coombs GH, Mottram JC (1997) Parasite proteinases and amino acid metabolism: possibilities for chemotherapeutic exploitation. Parasitology 114:S61-S80
Criado AF, Armas SC, Gimenez CP, Casado NE, Jimenez AG, Rodriguez FC (1992) Proteolytic enzymes from Trichinella spiralis larvae. Vet Parasitol 45:133–140
De Armas-Serra C, Gimenez-Pardo C, Jimenez-Gonzalez A, Bernadina WE, Rodriguez-Caabeiro F (1995a) Purification and preliminary characterization of a protease from the excretion-secretion products of Trichinella spiralis muscle-stage larvae. Vet Parasitol 59:157–168
De Armas-Serra C, Gimenes-Pardo C, Bernadina WE, Rodriguez-Caabeiro F (1995b) Antibody response to a protease secreted by Trichinella spiralis muscle larvae. Parasitol Res 81:540–542
Gamble HR, Fetterer RH, Mansfield LS (1996) Developmentally regulated zinc metalloproteinases from third- and fourth-stage larvae of the ovine nematode Haemonchus contortus. J Parasitol 82:197–202
Haffner A, Guilavogui AZ, Tischendorf FW, Brattig NW (1998) Onchocerca volvulus: Microfilariae secrete elastinolytic and males nonelastinolytic matrix-degrading serine and metalloproteinases. Exp Parasitol 90:26–33
Hill DE, Gamble HR, Rhoads ML, Fetterer RH, Urban JF (1993) Trichuris suis: A zinc metalloprotease from culture fluids of adult parasites. Exp Parasitol 77:170–178
Jiang W, Bond JS (1992) Families of metalloendopeptidases and their relationship. FEBS Lett 312:110–114
Ko RC, Mak CH (1999) Trichinellosis as a model of new frontier research on parasitic infections. Int Med Res J 3:1–11
Ko RC, Yeung MHF (1989) Specificity of ES antigens in detection of Trichinella spiralis antibodies in Chinese pigs. Trop Biomed 6:99–111
Lai YY (1996) Identification and characterization of proteolytic enzymes in Trichinella spp. M.Phil thesis. Department of Zoology, Univerisity of Hong Kong, Hong Kong
Lai YY, Ko RC (1994) A neutral metallo-protease from Trichinella spiralis infective larvae. Eighth International Congress of Parasitology, Izmir, Turkey, Abstract no. 192
McKerrow JH (1989) Parasite proteinases. Exp Parasitol 68:111–115
Moczon T, Wranicz M (1999) Trichinella spiralis: proteinases in the larvae. Parasitol Res 85:47–58
Nagano I, Wu Z, Nakada T, Matsuo A, Takahashi Y (2001) Molecular cloning and characterization of a serine proteinase inhibitor from Trichinella spiralis. Parasitology 123:77–83
Pei D, Weiss SJ (1995) Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature 375:244–247
Pendás AM, Knäuper V, Puente XS, Llano E, Mattei MG, Apte S, Murphy G, Lόpez-Otín C (1997) Identification and characterization of a novel human matrix metalloproteinase with unique structural characteristics, chromosomal location and tissue distribution. J Biol Chem 272:4281–4286
Polzer M, Taraschewski H (1993) Identification and characterization of the proteolytic enzymes in the developmental stages of the eel-pathogenic nematode Anguillicola crassus. Parasitol Res 79:24–27
Shapiro SD (1998) Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol 10:602–608
Stöcker W, Zwilling R (1995) Astacin. Methods in Enzymol 248:305–325
Stöcker W, Grams F, Baumann U, Reinemer P, Gomis-Ruth FX, Mckay DB, Bode W (1995) The metzincins. Topological and sequential relations between the astacins, adamalysins, serralysins and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci 4:823–840
Takamoto M, Sugane K (1993) Trichinella spiralis infective L1 larvae secrete serine proteinase(s). Jpn J Parasitol 42:277–294
Todorova VK (2000) Proteolytic enzymes secreted by larval stage of the parasitic nematode Trichinella spiralis. Folia Parasitol 47:141–145
Todorova VK, Knox DP, Kennedy MW (1995) Proteinases in the excretory/secretory products (ES) of adult Trichinella spiralis. Parasitology 111:201–208
Trap C, Le Rhun D, Roman T, Liu MY, Perret C, Boireau P (2000) Molecular cloning and analysis of a common stage Trichinella spiralis serine protease gene. Proceedings of the 10th International Conference on Trichinellosis, Fontainebleau, France, pp 39
van Wart HE, Birkedal-Hansen H (1990) The cysteine switch: a principle of regulation of metalloproteinase gene family. Proc Natl Acad Sci U S A 87:5578-5582
Yan L, Leontovich A, Fei K, Sarras MP (2000) Hydra metalloproteinase 1: a secreted astacin metalloproteinase whose apical axis expression is differentially regulated during head regeneration. Dev Biol 219:115–128
Yang M, Murray MT, Kurkinen M (1997) A novel matrix metalloproteinase gene (XMMP) encoding vitronectin-like motifs is transiently expressed in Xenopus laevis early embryo development. J Biol Chem 272:13527–13533
Yasumasu S, Yamada K, Adasaka K, Misunaga K, Iuchi I, Shimada H, Yamagami K (1992) Isolation of cDNAs for LCE and HCE, two constituent proteases of the hatching enzyme of Oryzlas latipes and concurrent expression of their mRNAs during development. Dev Biol 153:250–258
Acknowledgements
This study was supported by a grant from the Hong Kong Research Grant Council (HKU 426/96 M) and the Conference and Research Grants of the University of Hong Kong to R.C.K. We are very grateful for the assistance of Ms. Y.Y.Y. Chung and Mr. R. Leung.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lun, H.M., Mak, C.H. & Ko, R.C. Characterization and cloning of metallo-proteinase in the excretory/secretory products of the infective-stage larva of Trichinella spiralis . Parasitol Res 90, 27–37 (2003). https://doi.org/10.1007/s00436-002-0815-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-002-0815-0