Abstract
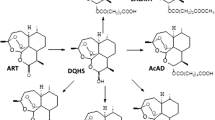
We recently found that the exposure of schistosomes in vitro to artemether plus haemin can lead to parasite death, while exposure to each compound singly had no effect. Since these observations might be relevant to understanding the mechanism of action of artemether against schistosomes, we conducted additional experiments. First, we performed a comparative appraisal of Schistosoma japonicum survival after incubation in two media, namely Hanks' balanced salt solution (HBSS) and RPMI 1640, supplemented with inactivated calf serum, antibiotics and different concentrations of artemether and/or haemin. Worm mortalities were consistently higher and occurred faster in HBSS when compared to RPMI 1640. Second, we investigated the behaviour of artemether in different chemical systems, including reduced glutathione or cysteine, in the presence of haemin or ferrous sulfate. Cleavage of the endoperoxide bridge of artemether occurred in all experiments, consistently forming five different products, of which one has not been described previously. Third, RPMI 1640 and HBSS media were supplemented with artemether and haemin in the presence of S. japonicum. The consumption of artemether in RPMI 1640 was much faster than in HBSS. Trace amounts of artemether and five free radical reaction products of artemether could be detected in RPMI 1640 after 24–48 h. In contrast, large amounts of artemether remained without the formation of free radical products in HBSS under the same conditions. These findings coincide with significantly higher schistosome mortalities employing HBSS instead of RPMI 1640. Our results suggest that it is artemether or an active metabolite thereof (most likely a carbon-centred free radical), rather than any free radical reaction product that is harmful for the worms. We speculate that schistosomes ingest artemether and cleave it in their gut. This cleavage is induced by haemin or another iron-containing molecule. These findings might be a further step forward in elucidating the mechanism of action of artemether against schistosomes.


Similar content being viewed by others
References
Cazelles J, Robert A, Meunier B (2002) Alkylating capacity and reaction products of antimalarial trioxanes after activation by a heme model. J Org Chem 67:609–619
Chen DJ, Fu LF, Shao PP, Wu FZ, Fan CZ, Shu H, Ren CS, Sheng XL (1980) Studies on antischistosomal activity of qinghaosu in experimental therapy (in Chinese). Zhong Hui Yi Xue Zha Zhi 80:422–428
China Cooperative Research Group on Qinghaosu and its Derivatives as Antimalarials (1982) The chemistry and synthesis of qinghaosu derivatives. J Tradit Chin Med 2:9–16
Haynes RK (2001) Artemisinin and derivatives: the future for malaria treatment? Curr Opin Infect Dis 14:719–726
Jefford CW (2001) Why artemisinin and certain synthetic peroxides are potent antimalarials. Implications for the mode of action. Curr Med Chem 8:1803–1826
Karbwang J, Na-Bangchang K, Thanavibul A, Molunto P (1998) Plasma concentrations of artemether and its major plasma metabolite, dihydroartemisinin, following a 5-day regimen of oral artemether, in patients with uncomplicated falciparum malaria. Ann Trop Med Parasitol 92:31–36
Klayman DL (1985) Qinghaosu (artemisinin): an antimalarial drug from China. Science 228:1049–1055
Le WJ, You JQ, Yang YQ, Mei JY, Guo HF, Yang HZ, Zang CW (1982) Studies on the efficacy of artemether in experimental schistosomiasis (in Chinese). Acta Pharmaceut Sin 17:187–193
Meshnick SR (2002) Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol 32:1655–1660
Meshnick SR, Thomas A, Ranz A, Xu CM, Pan HZ (1991) Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol Biochem Parasitol 49:181–189
Meshnick SR, Taylor TE, Kamchonwongpaisan S (1996) Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Rev 60:301–315
N'Goran EK, Utzinger J, Gnaka HN, Yapi A, N'Guessan NA, Kigbafori SD, Lengeler C, Chollet J, Xiao SH, Tanner M (2003) Randomized, double-blind, placebo-controlled trial of oral artemether for the prevention of patent Schistosoma haematobium infections. Am J Trop Med Hyg 68:24–32
Nosten F, Brasseur P (2002) Combination therapy for malaria: the way forward? Drugs 62:1315–1329
Olliaro PL, Haynes RK, Meunier B, Yuthavong Y (2001) Possible modes of action of the artemisinin-type compounds. Trends Parasitol 17:122–126
Posner GH, Meshnick SR (2001) Radical mechanism of action of the artemisinin-type compounds. Trends Parasitol 17:266–267
Posner GH, Oh CH (1992) A regiospecifically oxygen-18 labelled 1,2,4-trioxane: a simple chemical model system to probe the mechanism(s) for the antimalarial activity of artemisinin (qinghaosu). J Am Chem Soc 114:8328–8329
Price RN (2000) Artemisinin drugs: novel antimalarial agents. Expert Opin Investig Drugs 9:1815–1827
Robert A, Benoit-Vical F, Dechy-Cabaret O, Meunier B (2001) From classical antimalarial drugs to new compounds based on the mechanism of action of artemisinin. Pure Appl Chem 73:1173–1188
Robert A, Coppel Y, Meunier B (2002) Alkylation of heme by the antimalarial drug artemisinin. Chem Commun 2002:414–415
Sibmooh N, Udomsangpetch R, Kijjoa A, Chantharaksri U, Mankhetkorn S (2001) Redox reaction of artemisinin with ferrous and ferric ions in aqueous buffer. Chem Pharm Bull 49:1541–1546
Utzinger J, N'Goran EK, N'Dri A, Lengeler C, Xiao SH, Tanner M (2000) Oral artemether for prevention of Schistosoma mansoni infection: randomised controlled trial. Lancet 355:1320–1325
Utzinger J, Xiao SH, N'Goran EK, Bergquist R, Tanner M (2001) The potential of artemether for the control of schistosomiasis. Int J Parasitol 31:1549–1562
Wang DY, Wu YL (2000) A possible antimalarial action mode of qinghaosu (artemisinin) series compounds. Alkylation of reduced glutathione by C-centred primary radicals produced from antimalarial compound qinghaosu and 12-(2,4-dimethoixyphenyl)-deoxoqinghaosu. Chem Commun 2000:2193–2194
Wang DY, Wu Y, Wu YL, Shan F (1999) Synthesis, iron(II)-induced cleavage and in vivo antimalarial efficacy of 10-(2-hydroxy-1-naphthyl)-deoxoqinghaosu(-deoxoartemisinin). J Chem Soc Perkin Trans 1:1827–1831
Wang DY, Wu YL, Wu Y, Liang J, Li Y (2001) Further evidence for the participation of primary carbon-centred free radicals in the antimalarial action of the qinghaosu (artemisinin) series of compounds. J Chem Soc Perkin Trans 1:605–609
Wu WM, Wu YK, Wu YL, Yao ZJ, Zhou CM, Li Y, Shan F (1998) Unified mechanistic framework for the Fe(II)-induced cleavage of qinghaosu and derivatives/analogues. The first spin-trapping evidence for the previously postulated secondary C-4 radical. J Am Chem Soc 120:3316–3325.
Wu YK, Yue ZY, Wu YL (1999) Interaction of qinghaosu (artemisinin) with cysteine sulfhydryl mediated by traces of non-heme iron. Angew Chem Int Ed 38:2580–2582
Wu YK, Yue ZY, Liu HH (2001) New insights into the degradation of qinghaosu (artemisinin) mediated by non-heme-iron chelates, and their relavance to the antimalarial mechanism. Helv Chim Acta 84:928–932
Xiao SH, Booth M, Tanner M (2000a) The prophylactic effects of artemether against Schistosoma japonicum infections. Parasitol Today 16:122–126
Xiao SH, Hotez PJ, Tanner M (2000b) Artemether, an effective new agent for chemoprophylaxis against schistosomiasis in China: its in vivo effect on the biochemical metabolism of the asian schistosome. Southeast Asian J Trop Med Public Health 31:724–732
Xiao SH, Chollet J, Utzinger J, Matile H, Mei JY, Tanner M (2001) Artemether administered together with haemin damages schistosomes in vitro. Trans R Soc Trop Med Hyg 95:67–71
Xiao SH, Tanner M, N'Goran EK, Utzinger J, Chollet J, Bergquist R, Chen MG, Zeng J (2002) Recent investigations of artemether, a novel agent for the prevention of schistosomiasis japonica, mansoni and haematobia. Acta Trop 82:175–181
Yang YQ, Xiao SH, Tanner M, Utzinger J, Chollet J, Wu JD, Guo J (2001) Histopathological changes in juvenile Schistosoma haematobium harboured in hamsters treated with artemether. Acta Trop 79:135–141
Yolles TK, Moore DV, De Giusti DL, Ripsom CA, Meleney HE (1947) A technique for the perfusion of laboratory animals for the recovery of schistosomes. J Parasitol 33:419–426
Acknowledgements
This investigation received financial support from the Nine Five-Year Key Research Program of China, the Swiss Tropical Institute (STI), and the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases. J. Utzinger acknowledges support from the Swiss National Science Foundation, the Centre for Health and Wellbeing at Princeton University and STI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, SH., Wu, YL., Tanner, M. et al. Schistosoma japonicum: in vitro effects of artemether combined with haemin depend on cultivation media and appraisal of artemether products appearing in the media. Parasitol Res 89, 459–466 (2003). https://doi.org/10.1007/s00436-002-0786-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-002-0786-1