Abstract.
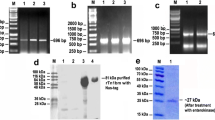
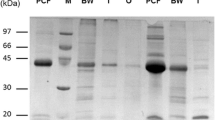
We recently reported the cDNA cloning and functional characterization of a novel transglutaminase (TGase) from the dog filarial parasite Dirofilaria immitis. D. immitis TGase (DiTG) has no sequence similarity with any other known TGase, but has significant similarity to protein disulfide isomerase (PDI)-related endoplasmic reticulum protein ERp60. In the present study, we further characterized the recombinant DiTG (rDiTG) and studied its role in the molting process of third-stage larvae. The enzymatic activity of rDiTG requires Ca2+, and the maximum activity was observed at a calcium concentration of 4 mM. Interestingly, the rDiTG was highly thermostable, with optimal activity observed at 55°C, similar to that seen with the native enzyme. Dithiothreitol (DTT) was not essential for enzyme activity. In fact, rDiTG was more active in the absence of DTT. The known inhibitors of TGase, such as monodansylcadaverine (MDC), cystamine and iodoacetamide, inhibited the TGase activity, but not the PDI activity, of rDiTG, demonstrating the dual activity of rDiTG. The TGase-specific pseudosubstrate, MDC, completely inhibited the molting of D. immitis L3 to L4 if present during the first 24–48 h of the molting process. Electron microscopic studies revealed that MDC-treated infective larvae failed to show separation between the L3 and L4 cuticles. The L4 cuticle and accompanying hypodermis were much thinner in MDC-treated worms than in controls. Using anti-rDiTG antiserum, the native DiTG antigen was localized in the hypodermis, afibrillar muscle cells and gut epithelium in adult male and female worms as well as developing embryos in the females.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Chandrashekar, R., Devarajan, E. & Mehta, K. Dirofilaria immitis: further characterization of the transglutaminase enzyme and its role in larval molting. Parasitol Res 88, 185–191 (2002). https://doi.org/10.1007/s00436-001-0520-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00436-001-0520-4