Abstract
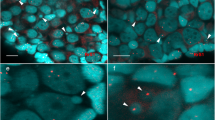
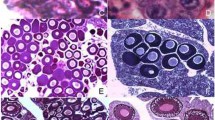
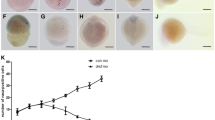
Pelophylax esculentus is the fertile hybrid of P. ridibundus and P. lessonae. During gametogenesis, one of the parental genomes is removed from the germ line cells, whereas the other one is clonally transmitted to the gametes. In hybrids, development of gonads is delayed in comparison with parental species. This may result from complex processes of genome elimination in female tadpoles at Gosner stages 28–46, potentially responsible for increased degeneration of germ cells in developing gonads from the very beginning of sexual differentiation to ovaries with diplotene oocytes, respectively. In this work, we revealed that germ cells died by apoptosis, as detected by expression of active caspase-3 using immunohistochemical method. The main group of degenerating germ cells was primary oogonia, however, in P. lessonae and P. ridibundus also secondary oogonia and diplotene oocytes were found. The number of degenerating germ cells was significantly higher in ovaries of P. esculentus. In hybrids, positive correlations were demonstrated between Gosner stage and gonadal volume, Gosner stage and the number of degenerating germ cells, gonadal volume and number of degenerating germ cells. These observations suggest that increased rate of apoptosis in germ cells, probably as the result of improper genome elimination, may be responsible for delayed maturation of ovaries in P. esculentus.



Similar content being viewed by others
References
Berger L (1983) Western palearctic water frogs (Amphibia, Ranidae): systematics, genetics and population compositions. Experientia 39:127–234
Berger L, Rybacki M, Hotz H (1994) Artificial fertilization of water frogs. Amphib Reptil 15:408–413
Chmielewska M, Symonowicz K, Pula B, Owczarek T, Podhorska-Okołów M, Ugorski M, Dzięgiel P (2015) Expression of metallothioneins I and II in kidney of doxorubicin-treated rats. Exp Toxicol Pathol 67:297–303. doi:10.1016/j.etp.2015.01.006
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Fujita J, Crane A, Souza M, Dejosez M, Kyba M, Flavell R, Thomson J, Zwaka T (2008) Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell 2:595–601. doi:10.1016/j.stem.2008.04.001
Gosner LK (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:513–543
Graf J-D, Polls-Pelaz M (1989) Evolutionary genetics of the Rana esculenta complex. In: Dawley R, Bogart J (eds) Evolution and ecology of unisexual vertebrates. New York State Museum, Albany, pp 289–302
Günther R, Plötner J (1990) Mating pattern in pure hybrid populations of water frogs, Rana kl. esculenta (Anura, Ranidae). Alytes 8:90–98
Hauswaldt S, Hoer M, Ogielska M, Christiansen D, Dziewulska-Szwajkowska D, Czernicka E, Vences M (2012) A simplified molecular method for distinguishing among species and ploidy levels in European water frogs (Pelophylax). Mol Ecol Resour 12:797–805
Hoffmann A, Plötner J, Pruvost NBM, Christiansen DG, Röthlisberger S, Choleva L, Mikulíček P, Cogălniceanu D, Sas-Kovács I, Shabanov D, Morozov-Leonov S, Reyer HU (2015) Genetic diversity and distribution patterns of diploid and polyploid hybrid water frog populations (Pelophylax esculentus complex) across Europe. Mol Ecol 24:4371–4391
Jakob C, Arioli M, Reyer HU (2010) Ploidy composition in all-hybrid frog populations in relation to ecological conditions. Evol Ecol Res 12(5):633–652
Joly P (2001) The future of the selfish hemiclone: a Neodarwinian approach to water frog evolution. Zoosystematics Evol 77:31–38
Lada GA, Borkin LJ, Vinogradov AE (1995) Distribution, population systems and reproductive behavior of green frogs (hybridogenetic Rana esculenta complex) in the central chernozem territory of Russia. Rus J Herpetol 2:46–57
Ogielska M (1994) Nucleus-like bodies in gonial cells of Rana esculenta [Amphibia, Anura] tadpoles—a putative way of chromosome elimination. Zool Pol 39:461–474
Ogielska M (2009) Development and reproduction of amphibian species, hybrids and polyploids. In: Ogielska M (ed) Reproduction of amphibians. Science Publisher, USA, pp 343–410
Ogielska M, Kotusz A (2004) Pattern and rate of ovary differentiation with reference to somatic development in anuran amphibians. J Morphol 259:41–54
Ogielska M, Wagner E (1990) Oogenesis and development of the ovary in European green frog, Rana ridibunda (Pallas). I. Tadpole stages until metamorphosis. Zool Jb Anat 120:211–221
Ogielska M, Wagner E (1993) Oogenesis and ovary development in the natural hybridogenetic water frog, Rana esculenta L. I tadpole stages until metamorphosis. Zool Jb Physiol 97:349–368
Ogielska M, Rozenblut B, Augustyńska R, Kotusz A (2010) Degeneration of germ line cells in amphibian ovary. Acta Zool Stockholm 91:319–327
Ogielska M, Kotusz A, Augustyńska R, Ihnatowicz J, Paśko Ł (2013) A stockpile of ova in the grass frog Rana temporaria is established once for the life span. Do ovaries in amphibians and in mammals follow the same evolutionary strategy? Anat Rec (Hoboken) 296:638–653. doi:10.1002/ar.22674
Rybacki M, Berger L (1994) Distribution and ecology of water frogs in Poland. Zool Pol 39:293–303
Rybacki M, Berger L (2001) Types of water frog populations (Rana esculenta complex) in Poland. Zool Reihe 77:51–57. doi:10.1002/mmnz.20010770109
Semlitsch RD, Schmiedehausen S et al (1996) Genetic compatibility between sexual and clonal genomes in local populations of the hybridogenetic Rana esculenta Complex. Evol Ecol 10:531–543
Socha M, Ogielska M (2010) Age structure, size and growth rate of water frogs from central European natural Pelophylax ridibundus—Pelophylax esculentus mixed populations estimated by skeletochronology. Amphib Reptil 31:239–250. doi:10.1163/156853810791069119
Tunner HG, Heppich-Tunner S (1991) Genome exclusion and two strategies of chromosome duplication in oogenesis of a hybrid frog. Naturwissenschaften 78:32–34
Wagner E, Ogielska M (1990) Oogenesis and development of the ovary in European green frog, Rana ridibunda (Pallas). II. Juvenile stages until adults. Zool Jb Anat (Jena) 120:223–231
Wagner E, Ogielska M (1993) Oogenesis and ovary development in the natural hybridogenetic water frog, Rana esculenta L. II. After metamorphosis until adults. Zool Jb Physiol (Jena) 97:369–382
Acknowledgements
This study was supported by the grant from Polish National Science Center No. 2012/07/B/NZ3/02563. Translation of publication was supported by Wrocław Centre of Biotechnology programme, the Leading National Research Centre (KNOW) for the years 2014–2018. We are grateful to Mrs. Ewa Serwa for help in immunohistochemical processing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. To carry out the research, consent was obtained from the local ethical commission in Wroclaw No. 7/2013. The acquisition of specimens of the species was accepted by license number DOP-oz.6401.02.2.2013.JRO.
Additional information
Paweł Szydłowski and Magdalena Chmielewska have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Szydłowski, P., Chmielewska, M., Rozenblut-Kościsty, B. et al. The frequency of degenerating germ cells in the ovaries of water frogs (Pelophylax esculentus complex). Zoomorphology 136, 75–83 (2017). https://doi.org/10.1007/s00435-016-0337-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-016-0337-4