Abstract
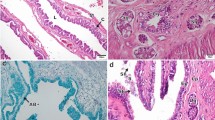
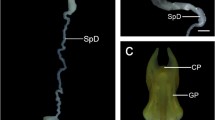
The capacity of storing sperm within the female reproductive tract occurs widely across vertebrate and invertebrate species. Although the type and position of spermathecae have been commonly used as a taxonomic character in Opisthopora, few studies have focused on the ultrastructural description of these interesting storage organs. This study is the first to report on the ultrastructure of the spermathecae and spermatozoa of Hormogaster elisae, an endemism of the central area of the Iberian Peninsula that presents two pairs of tubular spermathecae. Light and electron microscopy showed that the spermathecae are full of highly packed spermatozoa embedded in an electron-dense substance. Two layers constitute the spermathecal wall. The outer layer consists of peritoneal cells, collagenous basal laminae at different levels, several layers of striated muscle, and numerous blood vessels. The inner layer is a monostratified epithelium of prismatic cells presenting long and abundant microvilli probably for the maintenance of a favorable environment for the spermatozoa. The epithelial cells show high activity, and three different types of secretions were detected: holocrine, merocrine, and apocrine, whose hypothetical function on nourishment and/or causing quiescence is discussed here. Although no phagocytotic processes were detected, some sperm cells were observed in digestive vesicles within the cytoplasm of the epithelial cells, and there was also evidence of active sperm entrance into the epithelium. A place for nourishment or a slaughterhouse? Probably both.







Similar content being viewed by others
References
Beese K, Armbruster GFJ, Beier K, Baur B (2009) Evolution of female sperm-storage organs in the carrefour of stylommatophoran gastropods. J Zool Syst Evol Res 47:49–60
Birkhead TR, Møller AP (1993) Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Biol J Linn Soc 50:295–311
Birkhead TR, Møller AP, Sutherland WJ (1993) Why do females make it so difficult for males to fertilize their eggs? J Theor Biol 161:51–60
Bojat NC, Sauder U, Haase M (2001) The spermathecal epithelium, sperm and their interactions in the hermaphroditic land snail Arianta arbustorum (Pulmonata, Stylommatophora). Zoomorphology 120:147–157
Brinkhurst RO, Jamieson BGM (1971). Aquatic oligochaeta of the world. Oliver and Boyd, Edinburgh
Butt KR, Nuutinen V (1998) Reproduction of the earthworm Lumbricus terrestris Linné after the first mating. Can J Zool 76:104–109
Dent JN (1970) The ultrastructure of the spermatheca in the red spotted newt. J Morphol 132:397–424
Díaz Cosín DJ, Ruiz MP, Ramajo M, Gutiérrez M (2006) Is the aestivation of the earthworm Hormogaster elisae a paradiapause? Inv Biol 125:250–255
Díaz Cosín DJ, Hernández P, Trigo D, Fernández R, Novo M (2009) Algunos aspectos del ciclo biológico del endemismo ibérico, Hormogaster elisae Álvarez, 1977 (Oligochaeta, Hormogastridae), en cultivos de laboratorio. Bol R Soc Esp Hist Nat Biol 103:49–57
Díaz Cosín J, Novo M, Fernández R (2011) Reproduction of earthworms: sexual selection and parthenogenesis. In: Karaka A (ed) Biology of earthworms. Springer, Berlin, pp 69–86
Dixon G (1915) Tubifex. In: Herdman WA (ed) LMBC memoirs on typical British marine plants and animals, vol 23. Williams and Norgate, London, pp 1–100
Eberhard WG (1985) Sexual selection and animal genitalia. Harvard University Press, Cambridge
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton, NJ
Edwards CA, Bohlen PJ (1996) Biology and ecology of earthworms, 3rd edn. Chapman and Hall, London
Eisen G (1874) New Englands och Canadas Lumbricides. Ofversigt af Kongliga Vetenskaps-Akademiens Forhandligar Stockholm 31(2):41–49
Fernández R, Bergmann P, Almodóvar A, Díaz Cosín DJ, Heethoff M (2011) Ultrastructural and molecular insights into three populations of Aporrectodea trapezoides (Dugés, 1828) (Oligochaeta, Lumbricidae) with different reproductive modes. Pedobiologia 54:281–290
Ferraguti M, Jamieson BGM (1984) Spermiogeness and spermatozoal ultrastructure in Hormogaster (Hormogastridae, Oligochaeta, Annelida). J Submicrosc cytol 16:307–316
Fleming TP (1981) The ultrastructure of the spermathecae of Tubifex tubifex (Annelida: Oligochaeta). J Zool Lond 193:129–145
Garvín MH, Trigo D, Hernández P, Díaz Cosín DJ (2003) Gametogenesis and reproduction in Hormogaster elisae (Oligochaeta, Hormogastridae). Inv Biol 122:152–157
Grove AJ (1925) On the reproductive processes of the earthworm, Lumbricus terrestris. Q J Microsc Sci 69:245–290
Grube E (1879) Zoology of Rodriguez. Annelida. Phil Trans R Soc London 168:554–556
Gutiérrez M, Jesús JB, Trigo D, Díaz Cosín DJ (2006) Is Hormogaster elisae (Oligochaeta, Hormogastridae) a predator of mite and springtails? Eur J Soil Biol 42:S186–S190
Hellriegel B, Bernasconi G (2000) Female-mediated differential sperm storage in a fly with complex spermathecae, Scatophaga stercoraria. Anim Behav 59:311–317
Hilario A, Young CM, Tyler PA (2005) Sperm storage, internal fertilization, and embryonic dispersal in vent and seep tubeworms (Polychaeta: Siboglinidae: Vestimentifera). Biol Bull 208:20–28
Holt WV, Lloyd RE (2010) Sperm storage in the vertebrate female reproductive tract: how does it work so well? Theriogenology 73:713–722
Jamieson BGM (1981) The ultrastructure of the oligochaeta. Academic Press, London, p 462
Jamieson BGM (1992) Oligochaeta. In: Harrison FW, Gardiner SL (eds) Microscopic anatomy of invertebrates, vol 7. Wiley-Liss, New York
Jamieson BGM (2001) Native earthworms of Australia (Megascolecidae, Megascolecinae). Science Publishers, Inc., Enfield
Jamieson BGM (2006) Non-leech Clitellata. In: Reproductive biology and phylogeny of annelida. Series Editor Jamieson BGM. Rouse G, Pleijel F (eds), vol 4. Chap. 8. Science Publishers, Enfield
Linnæus C (1758) Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. pp [1–4], 1–824. Holmiæ. (Salvius)
McCoy F (1878) Megascolides australis (McCoy). The giant earth-worm. Prodromus Zool Vic 1:21–25
Meyer WJ, Bowman H (1994) Mating period and cocoon production in Eisenia fetida. The 5th international symposium on earthworm ecology Columbus Ohio, 5–9, p 128
Novo M, Almodóvar A, Díaz Cosín DJ (2009) High genetic divergence of hormogastrid earthworms (Annelida, Oligochaeta) in the central Iberian Peninsula: evolutionary and demographic implications. Zool Scripta 38:537–552
Novo M, Almodóvar A, Fernández R, Trigo D, Díaz Cosín DJ (2010a) Cryptic speciation of hormogastrid earthworms revealed by mitochondrial and nuclear data. Mol Phylogenet Evol 56:507–512
Novo M, Almodóvar A, Fernández R, Gutiérrez M, Díaz Cosín DJ (2010b) Mate choice of an endogeic earthworm revealed by microsatellite markers. Pedobiologia 53:375–379
Novo M, Almodóvar A, Fernández R, Giribet G, Díaz Cosín DJ (2011) Understanding the biogeography of a group of earthworms in the Mediterranean basin—the phylogenetic puzzle of Hormogastridae (Clitellata: Oligochaeta). Mol Phylogenet Evol 61:125–135
Qiu JP, Bouché MB (1998) Contribution to the taxonomy of Hormogastridae (Annelida: Oligochaeta) with description of new species from Spain. Doc Pedozool Integrol 4:164–177
Rafinesque CS (1820) Ichthyologia Ohiensis. Reprinted edition: Burrows Brothers Co., Cleveland, 1899
Richards KS, Fleming TP (1982) Spermatozoal phagocytosis by the spermathecae of Dendrobaena subrubicunda and other lumbricids (Oligochaeta, Annelida). Int J Invert Rep 5:233–241
Rosa D (1887) Hormogaster redii n. g., n. sp. Bollettino dei Musei di Zoologia ed Anatomia comparata della Universita di Torino 32(II)
Savigny JC (1826) In Cuvier, Analyse des travaux de l’Academie Royale des Sciences pendant l’année 1821, partie physique. Mem Acad Roy Sci Inst Fr 5:176–184
Teisaire ES, Roldán IA (1995) Ultrastructure and histochemistry of the spermatheca of Amynthas Kinberg and Metaphire Sims and Easton (Oligochaeta: Megascolecidae). Comun Biol 13:169–181
Valle JV, Moro RP, Garvín MH, Trigo D, Díaz Cosín DJ (1997) Annual dynamics of the earthworm Hormogaster elisae (Oligochaeta, Hormogastridae) in Central Spain. Soil Biol Biochem 29:309–312
Vanpraagh BD (1995) Reproductive biology of Megascolides australis McCoy (Oligochaeta: Megascolecidae). Aust J Zool 43:489–507
Varuta AT, More NK (1972) Cytochemical study of mucus and mucus secreting cells in spermathecae of the earthworms, Pheretima elongata (Perrier) and Hoplochaetella powelli (Michaelsen). Ind Exp Biol 10:239–241
Vyas I, Dev B (1972) Histochemical localization of alkaline phosphatase in the spermathecae of the earthworm, Barogaster annandalei (Stephenson). Acta Histochem 42:344–350
Acknowledgments
We are indebted to Rosa Fernández for field assistance and advice on sample preparation. We also thank Carolyn Marks and Adam Graham for their constant availability and help on sample processing and TEM imaging in the Center for Nanoscale Systems (CNS) at Harvard University. M.N. was supported by a Grant from Fundación Caja Madrid and A.R. by a Marie Curie Outgoing Fellowship. This research was funded by internal MCZ funds to G.G. and CGL2010/16032 from the Spanish Ministry of Science and Innovation to D.J.D.C. and C.R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Bartolomaeus.
Marta Novo and Ana Riesgo equally contributed to this work.
Rights and permissions
About this article
Cite this article
Novo, M., Riesgo, A., Roldán, C. et al. A place for nourishment or a slaughterhouse? Elucidating the role of spermathecae in the terrestrial annelid Hormogaster elisae (Clitellata: Opisthopora: Hormogastridae). Zoomorphology 131, 171–184 (2012). https://doi.org/10.1007/s00435-012-0151-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-012-0151-6