Abstract
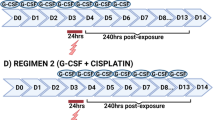
Peptide growth factors involved in the regulation of haematopoiesis (HPGF), for example granulocyte-colony-stimulating factor (G-CSF) and granulocyte/macrophage-colony-stimulating factor (GM-CSF), are of clinical importance in the treatment of testicular germ cell tumour (GCT) patients with modern chemotherapy regimens since they ameliorate chemotherapy-induced neutropenia. Aberrant expression of and/or response to HPGF has been reported in several solid tumour types although no data are available on GCT with the exception of those on stem cell factor (SCF). The aims of this pre-clinical study were twofold: (1) to screen a panel of human non-seminomatous (NS)GCT for the production of HPGF and (2) to test the effects of G-CSF or SCF on the growth of NSGCT cell lines in vitro, and on the growth kinetics of two human NSGCT xenograft models. HPGF concentrations in cell culture supernatant from 11 NSGCT cell lines growing under routine culture conditions were measured by enzyme-linked immunosorbent assay. The growth kinetics of cell lines was quantified in vitro using the sulphorhodamine B assay. The growth kinetics of nude mouse NSGCT xenografts was followed by measuring tumour volumes every 2–3 days over days 1–30, following daily subcutaneous injection of nude mice (days 1–14). The cell lines produced G-CSF (1/11 cell lines), GM-CSF (2/11), SCF (2/11), M-CSF (6/11), and interleukin-6 (9/11). Growth stimulation of cell line H12.1 by SCF was observed in vitro, but no statistically significant differences in NSGCT xenograft tumour volume (V T) or relative V T (rV T) in treated groups were observed on days 14 or 29 compared to the control. The change in rV T of H12.1 xenografts treated with G-CSF alone compared to control (rV T/rV T,c) was 0.96 on day 29. The values for rV T/rV T,c for H12.1 xenografts treated with G-CSF in combination with low- or high-dose SCF were, respectively, 1.67 or 1.7 compared to 1.19 for SCF-treated mice. The results are in agreement with clinical data to date where no observations have been reported of stimulation or inhibition of tumours in patients receiving treatment with G-CSF. Before any clinical trials are initiated in GCT patients treated with G-CSF in combination with SCF, further pre-clinical experiments with this tumour type are recommended to investigate this phenomenon further in a greater number of NSGCT cell lines in vitro and in vivo and with a wider range of SCF/G-CSF schedules. The potential relevance of secretion of HPGF in NSGCT cell lines in vitro to the pathobiology of GCT in patients is also a subject of interest for future research.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 12 May 1997 / Accepted: 19 April 1998
Rights and permissions
About this article
Cite this article
Dunn, T., Schmoll, HJ., Rie, C. et al. Production and pre-clinical significance of haematopoietic peptide growth factors (HPGF) in human non-seminomatous germ cell tumour cell lines. J Cancer Res Clin Oncol 124, 435–443 (1998). https://doi.org/10.1007/s004320050196
Issue Date:
DOI: https://doi.org/10.1007/s004320050196