Abstract
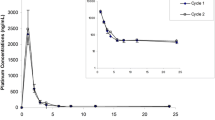
The pharmacokinetics of cis-diamminedichloro platinum(II) (cisplatin), given as a continuous infusion with concurrent radiotherapy to patients with locally advanced inoperable non-small-cell lung carcinoma, was investigated in 16 cases. The regimen, repeated for 6 consecutive weeks, consisted of weekly 10-Gy radiotherapy given in five fractions from Monday to Friday, and concurrent 100-h infusion of cisplatin delivered at a daily dose of 4 mg/m2 by a central venous catheter and a portable pump. Throughout the weeks of therapy the platinum levels were determined in plasma and in ultrafiltered plasma by respectively inductively coupled plasma atomic emission spectrometry and inductively coupled plasma mass spectrometry. Mean levels of platinum in plasma ([Pt]tot ) increased from the 1st to the 6th week of infusion, while mean levels of platinum in ultrafiltered plasma ([Pt]uf ), 110 μg/l, showed no marked variation throughout the therapy. [Pt]uf ranged from 16% to 22% of the total Pt. Mean levels of Pt in ultrafiltered plasma were of the same order of magnitude as those found to be active invitro as radiopotentiators. Pt decay levels were measured for 24 h at the end of the 1st and 5th weeks of infusion, allowing the calculation of the Pt half-life and the area under the decay curves. The mean value of the area under the decay curve, plotting [Pt]tot against time (AUC), in the range 0–24 h from the end of the 5th week of infusion, was about twice that from the end of the 1st week; by contrast, the mean AUC values did not vary for the [Pt]uf against time curves. The mean values of the α half-life of Pt in the ultrafiltered plasma were in accordance with those published in the literature; however, an unexpected very long β half-life was found (more than 100 h). Thus it was suggested that Pt species other than free cisplatin were present in the ultrafiltered plasma; such species probably involve metal bound to low-molecular-mass proteins. Throughout the therapy, the toxic effects in all patients were negligible, and 75% of them had an objective locoregional reduction of disease. In only 2 cases was progression of disease observed within the irradiated area. On the basis of these data, it can be concluded that cisplatin at a level of 110 μg/l in the ultrafiltered plasma, in the reported scheme of continuous intravenous infusion, has an enhancing effect on radiation and avoids concentration peaks of platinum not bound to protein.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 4 August 1997 / Accepted: 30 September 1997
Rights and permissions
About this article
Cite this article
Morazzoni, F., Canevali, C., Zucchetti, M. et al. cis-Diamminedichloroplatinum(II) given in low-dose continuous infusion with concurrent radiotherapy to patients affected by inoperable lung carcinoma: a pharmacokinetic approach. J Cancer Res Clin Oncol 124, 37–43 (1998). https://doi.org/10.1007/s004320050131
Issue Date:
DOI: https://doi.org/10.1007/s004320050131