Abstract
Background
The aim of this study was to determine the drug profile of patients with non-small cell lung cancer (NSCLC) and to identify potential drug–drug interactions (PDDIs) during hospitalization. In particular, PDDIs in categories X and D were determined.
Methods
This retrospective cross-sectional study was conducted in the oncology services of a university hospital between 2018 and 2021. PDDIs were evaluated using Lexicomp Drug Interactions® software included in UpToDate®.
Results
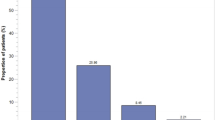
A total of 199 patients were included in the study. Polypharmacy was present in 92.5% of the patients and the median (min–max) number of drugs used was 8 (2–16). 32% of the patients had D and X PDDIs. A total of 16 PDDIs at risk grade X were found in 15 (7.5%) patients. A total of 81 PDDIs of risk grade D were found in 54 (27.1%) patients and a total of 276 PDDIs of risk grade C were identified in 97 (48.7%) patients. Anticancer drugs (p = 0.008), opioids (p = 0.046), steroids (p = 0.003), 5-HT3 receptor antagonists (p = 0.012), aprepitant (p = 0.025) and antihistamines (p < 0.001) were statistically more frequent among patients with PDDIs than among those without.
Conclusion
The results of our study indicated that polypharmacy and PDDIs are common in hospitalized patients with NSCLC cancer. The monitoring of medications is critical for maximizing therapeutic effects and minimizing side effects related to PDDIs. As a part of multidisciplinary team, clinical pharmacists can contribute significantly to preventing, detecting and managing PDDIs.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abunahlah N, Sancar M, Dane F, Özyavuz MK (2016) Impact of adherence to antiemetic guidelines on the incidence of chemotherapy-induced nausea and vomiting and quality of life. Int J Clin Pharm 38:1464–1476
Ajimura CM, Jagan N, Morrow LE, Malesker MA (2018) Drug interactions with oral inhaled medications. J Pharm Technol 34(6):273–280
Akiyoshi T, Ito M, Murase S, Miyazaki M, Guengerich FP, Nakamura K, Yamamoto K, Ohtani H (2013) Mechanism-based inhibition profiles of erythromycin and clarithromycin with cytochrome P450 3A4 genetic variants. Drug Metab Pharmacokinet 28(5):411–415
Alkan A, Yaşar A, Karcı E, Köksoy EB, Ürün M, Şenler FÇ, Ürün Y, Tuncay G, Ergün H, Akbulut H (2017) Severe drug interactions and potentially inappropriate medication usage in elderly cancer patients. Supp Care Cancer 25:229–236
Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB (2008) Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett 267(1):133–164
BCCA protocol (2021) non-small cell lung cancer Available from: http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Lung/LUAJPC_Protocol.pdf Accessed May 2023
Bektay MY, Seker Z, Eke HK, Turk HM, Izzettin FV (2022) Comparison of different decision support software programs in perspective of potential drug–drug interactions in the oncology clinic. J Oncol Pharm Pract. https://doi.org/10.1177/10781552221104814
Bibi R, Azhar S, Iqbal A, Jabeen H, Kalsoom U-E, Iqbal MM, Nazeer M (2021) Prevalence of potential drug-drug interactions in breast cancer patients and determination of their risk factors. J Oncol Pharm Pract 27(7):1616–1622
Björkman IK, Fastbom J, Schmidt IK, Bernsten CB (2002) Drug—drug interactions in the elderly. Ann Pharmacother 36(11):1675–1681
Blower P, De Wit R, Goodin S, Aapro M (2005) Drug–drug interactions in oncology: why are they important and can they be minimized? Crit Rev Oncol 55(2):117–142
Cicin I, Oksuz E, Karadurmus N, Malhan S, Gumus M, Yilmaz U, Cansever L, Cinarka H, Cetinkaya E, Kiyik M (2021) Economic burden of lung cancer in Turkey: a cost of illness study from payer perspective. Health Econ Rev 11(1):1–12
Dando TM, Perry CM (2004) Aprepitant: a review of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs 64:777–794
Delpeuch A, Leveque D, Gourieux B, Herbrecht R (2015) Impact of clinical pharmacy services in a hematology/oncology inpatient setting. Anti Res 35(1):457–460
Faqeer N, Mustafa N, Abd Al-jalil N, Qur’an T, (2021) Impact of clinical pharmacists in an inpatient medical oncology service: a prospective study at a comprehensive cancer center in Jordan. J Oncol Pharm Pract 27(4):897–901
Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J (2006) Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24(28):4539–4544
Hines LE, Murphy JE (2011) Potentially harmful drug–drug interactions in the elderly: a review. Am J Geriatr Pharmacother 9(6):364–377
Hong S, Lee JH, Chun EK, Kim KI, Kim JW, Kim SH, Lee YG, Hwang IG, Kim JY, Koh SJ (2020) Polypharmacy, inappropriate medication use, and drug interactions in older Korean Patients with cancer receiving first-line palliative chemotherapy. Oncologist 25(3):e502–e511
International Agency for Research on Cancer 2012 Estimated cancer incidence, prevalence and mortality worldwide in 2012. http://globocan.iarc.fr/Pages/online.aspx Accessed April 2023.
Jarzyna D, Jungquist CR, Pasero C, Willens JS, Nisbet A, Oakes L, Dempsey SJ, Santangelo D, Polomano RC (2011) American society for pain management nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Manag Nurs 12(3):118–145
Jazbar J, Locatelli I, Horvat N, Kos M (2018) Clinically relevant potential drug–drug interactions among outpatients: a nationwide database study. Res Social Adm Pharm 14(6):572–580
Laban A, Birand N, Chukwunyere U, Abdi A, Başgut B (2021) Evaluation of drug-drug interactions in cancer patients treated at a university hospital in North Cyprus using two interaction databases. Niger J Clin Pract 24(7):1067–1071
Lakkad M, Martin B, Li C, Harrington S, Dayer L, Painter JT (2023) The use of gabapentinoids and opioids and risk of developing opioid-induced respiratory depression among older breast cancer survivors with neuropathic pain. J Cancer Surviv. https://doi.org/10.1007/s11764-023-01338-9
McIntyre A, Ganti AK (2017) Lung cancer—a global perspective. J Surg Oncol 115(5):550–554
Mertens WC, Higby DJ, Brown D, Parisi R, Fitzgerald J, Benjamin EM, Lindenauer PK (2003) Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol 21(7):1373–1378
Moghaddas A, Adib-Majlesi M, Sabzghabaee AM, Hajigholami A, Riechelmann R (2021) Potential drug–drug Interactions in hospitalized cancer patients: a report from the Middle-East. J Oncol Pharm Pract 27(1):46–53
Nachimuthu S, Assar MD, Schussler JM (2012) Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf 3(5):241–253
Occhipinti M, Brambilla M, Galli G, Manglaviti S, Prelaj A, Ferrara R, De Toma A, Beninato T, Zattarin E, Proto C (2021) 133P drug-drug interactions (DDIs) in non-small cell lung cancer during chemotherapy-immunotherapy treatment. J Thorac Oncol 16(4):S769–S770
Park JW, Roh JL, Lee SW, Kim SB, Choi SH, Nam SY, Kim SY (2016) Effect of polypharmacy and potentially inappropriate medications on treatment and posttreatment courses in elderly patients with head and neck cancer. J Cancer Res Clin Oncol 142:1031–1040
Patel P, Leeder JS, Piquette-Miller M, Dupuis LL (2017) Aprepitant and fosaprepitant drug interactions: a systematic review. Br J Clin Pharmacol 83(10):2148–2162
Rahman HH, NiemannD M-M (2022) Association between asthma, chronic bronchitis, emphysema, chronic obstructive pulmonary disease, and lung cancer in the US population. Environ Sci Pollut Res 30(8):1–12
Ramsdale E, Mohamed M, Yu V, Otto E, Juba K, Awad H, Moorthi K, Plumb S, Patil A, Vogelzang N (2022) Polypharmacy, potentially inappropriate medications, and drug-drug interactions in vulnerable older adults with advanced cancer initiating cancer treatment. Oncologist 27(7):e580–e588
Rashdan S, Yang H, Le T, Selby C, Gerber DE, Alvarez CA (2021) Prevalence and significance of potential pharmacokinetic drug–drug interactions among patients with lung cancer: implications for clinical trials. Clin Drug Investig 41:161–167
Reimche L, Forster AJ, Van Walraven C (2011) Incidence and contributors to potential drug-drug interactions in hospitalized patients. J Clin Pharmacol 51(7):1043–1050
Riechelmann RP, Del Giglio A (2009) Drug interactions in oncology: how common are they? Ann Oncol 20(12):1907–1912
Rigas JR (2004) Taxane-platinum combinations in advanced non-small cell lung cancer: a review. Oncologist 9(S2):16–23
Roblek T, Vaupotic T, Mrhar A, Lainscak M (2015) Drug-drug interaction software in clinical practice: a systematic review. Eur J Clin Pharmacol 71:131–142
Rowinsky EK, Gilbert M, McGuire W, Noe D, Grochow L, Forastiere A, Ettinger D, Lubejko B, Clark B, Sartorius S (1991) Sequences of taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol 9(9):1692–1703
Ruths S, Viktil KK, Blix HS (2007) Classification of drug-related problems. Tidsskr nor Laegeforen 127:3073–3076
Scripture CD, Figg WD (2006) Drug interactions in cancer therapy. Nat Rev Cancer 6(7):546–558
Strandell J, Wahlin S (2011) Pharmacodynamic and pharmacokinetic drug interactions reported to VigiBase, the WHO global individual case safety report database. Eur J Clin Pharmacol 67:633–641
Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A (2021) Epidemiology of lung cancer. Contemp Oncol 25(1):45–52
Turner JP, Jamsen KM, Shakib S, Singhal N, Prowse R, Bell JS (2016) Polypharmacy cut-points in older people with cancer: how many medications are too many? Support Care Cancer 24:1831–1840
Umar RM, Apikoglu-Rabus S, Yumuk PF (2020) Significance of a clinical pharmacist-led comprehensive medication management program for hospitalized oncology patients. Int J Clin Pharm 42:652–661
Uptodate Lexicomp Drugs & Drug Interaction Available from: https://www.uptodate.com/home/drugs-drug-interaction Accessed January 2023
US Food and Drug Administration (2012) Docetaxel injection, solution for intravenous infusion. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/201525s002lbl.pdf Accessed May 2023
US Food and Drug Administration (2020) Paclitaxel injection. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf. Accessed May 2023
Vella-Brincat J, Macleod A (2007) Adverse effects of opioids on the central nervous systems of palliative care patients. J Pain Palliat Care Pharmacother 21(1):15–25
Yancik R (2005) Population aging and cancer: a cross-national concern. Cancer J 11(6):437–441
Acknowledgements
The authors would like to give a special thanks to Prof. Dr. Bilgen Başgut for providing help and statements that greatly improved the manuscript.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
AA and EK: conceived the study, AA: designed the study, analyzed data and wrote the manuscript. AA, EK and TD: collected data. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethics committee approval was obtained from Suleyman Demirel Clinical Research Ethics Committee (Approval No: 272, Date: 28.09.2022).
Informed consent
Since it was a retrospective study, informed consent were not obtainable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albayrak, A., Düzenli, T. & Kayıkçıoğlu, E. Potential drug–drug interactions in patients with non-small cell lung cancer at a university hospital in Turkey. J Cancer Res Clin Oncol 149, 9621–9627 (2023). https://doi.org/10.1007/s00432-023-04890-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04890-0